Iron(II) ions react with phenanthroline (C 12 H 8 N 2 ) to form a complex ion
Question:
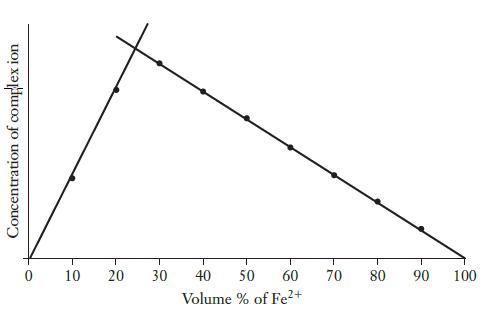
Iron(II) ions react with phenanthroline (C12H8N2) to form a complex ion with the general formula Fex(C12H8N2)y 2x+. A student uses the method of continuous variation to determine the number of phenanthroline molecules bound to each iron ion (i.e., the values of x and y in the formula). The complex ion is strongly colored, so its concentration can be monitored using a spectrometer. Working with 2.4 × 10-3 M solutions of both Fe3+and C12H8N2, the data shown in the graph below were obtained. What is the correct formula for the complex ion?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





