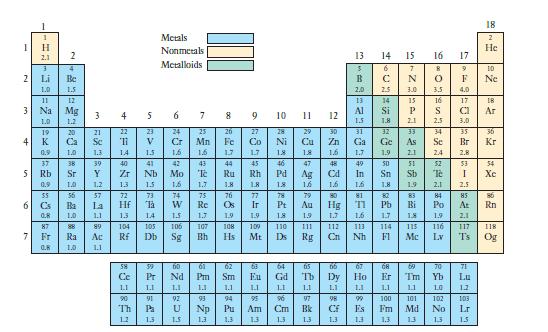
Nearly all of the other elements form binary compounds with hydrogen. Based on the electronegativity values shown
Question:
Nearly all of the other elements form binary compounds with hydrogen. Based on the electronegativity values shown in Figure 7.7, with what category of elements will hydrogen form bonds in which the hydrogen atom carries a partial negative charge? With what category of elements will hydrogen form bonds in which the hydrogen atom carries a partial positive charge? How does hydrogen’s electronegativity value help explain its ability to form both of these types of bonds?
Figure 7.7
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





