Once ozone forms in photochemical smog, there are a number of reactions by which it is subsequently
Question:
Once ozone forms in photochemical smog, there are a number of reactions by which it is subsequently destroyed. One such reaction occurs when ozone molecules encounter hydroxyl radicals:
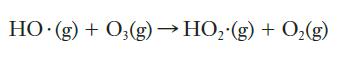
The following values for the rate constant, k, for this reaction were measured in experiments at various temperatures:
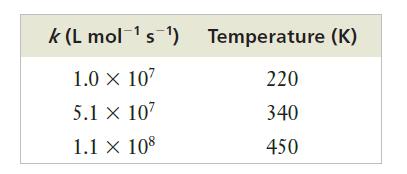
(a) Does this reaction display Arrhenius behavior?
(b) Estimate the activation energy from these data.
Strategy To determine whether or not the reaction displays Arrhenius behavior, we will need to compare the data to the model represented by the Arrhenius equation. To do this, we plot ln k versus 1/T. If the plotted points form a straight line, the slope of the line is –Ea/R. So, we can determine the activation energy from that slope.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





