Refer to the data and energy-level diagram shown in Problem 6.26, and find the wavelength of light
Question:
Refer to the data and energy-level diagram shown in Problem 6.26, and find the wavelength of light that would be emitted when a hydrogen atom undergoes the transition from the state labeled as n = 4 to the state labeled as n = 2. Express your answer in nm.
Data from problem 6.26
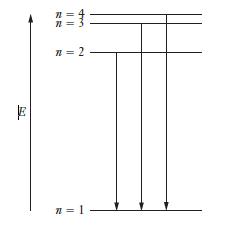
Transcribed Image Text:
n 二 n = 2 n = 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)

Answered By

Ayush Mishra
I am a certified online tutor, with more than 3 years of experience in online tutoring. My tutoring subjects include: Physics, Mathematics and Mechanical engineering. I have also been awarded as best tutor for year 2019 in my previous organisation. Being a Mechanical Engineer, I love to tell the application of the concepts of science and mathematics in the real world. This help students to develop interest and makes learning fun and easy. This in turn, automatically improves their grades in the subject. I teach students to get prepared for college entry level exam. I also use to teach undergraduate students and guide them through their career aim.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
The first four energy levels for the hydrogen atom are shown in the diagram below. The lowest energy state is labeled E 1 , and the higher states are labeled E 2 , E 3 , and E 4 , respectively. When...
-
The visible lines in the hydrogen atom emission spectrum arise from transitions with a final state with n = 2. In what spectral region should we expect to find transitions that have a final state of...
-
A hydrogen atom undergoes a transition from a 2p state to the Is ground state. In the absence of a magnetic field, the energy of the photon emitted is 122 nm. The atom is then placed in a strong...
-
? Fisheries Bhd is a small listed company involves in fishing and selling fish to the public via retail outlets. The company owns a building that was acquired in early September 2014 for RM1,200,000,...
-
If you were conducting a merger analysis, would you give the multiples method or one of the DCF methods (that is, the APV or corporate valuation model) more weight in your decision? Explain.
-
Nicholas is the facilities manager for Green Market Groceries. The store is remodeling and wants to determine which brand of freezer to use for its frozen goods section. The freezers vary by size,...
-
What do you know about the mathematical value of a project's internal rate of return under each of the following conditions? a. The present worth of the project is greater than 0 . b. The present...
-
The following information pertains to Worthy Video Company. 1. Cash balance per bank, July 31, $7,293. 2. July bank service charge not recorded by the depositor $28. 3. Cash balance per books, July...
-
Discuss the importance of an object-oriented database. How does it differ from the relational model?
-
Using only the periodic table, rank the following elements in order of increasing ionization energy: Br, F, Ga, K, and Se. Strategy We want to arrange the elements from the smallest ionization energy...
-
Carbon can be detected in an X-ray fluorescence experiment by monitoring the emission at a wavelength of 4.47 nm. What is the frequency of this light? Strategy We know that wavelength and frequency...
-
Green Company has the following post-closing trial balance on December 31, 2016: The companys accounting department has gathered the following budgeting information for the first quarter of 2017:...
-
Two types of football helmets, padded and suspension were compared to determine which had the lowest rate of impact damage. Forty helmets of each type were randomly selected and subjected to an...
-
The equilibrium price for parking in the downtown area of a large city is about $35 a day, this is represented by Pe in the graph above. The city council is looking at ways to resolve parking...
-
A Japanese automaker produces cars at a plant in Ohio. What is added to GDP?
-
Martha is covering kitchen shelves with shelving paper. She has 6 shelves that are each 1(3)/(4) feet long. She buys 13(1)/(4) feet of shelving paper of the correct width.
-
Toulouse, aged 85, has $300,000 superannuation balance all of which is taxable. She also has a $700,000 life insurance policy incorporated within her superannuation policy. Toulouse does not have any...
-
The following questions are used in the Kaplan CPA Review Course to study leases while preparing for the CPA examination. Determine the response that best completes the statements or questions. 1. A...
-
Diamond Walker sells homemade knit scarves for $25 each at local craft shows. Her contribution margin ratio is 60%. Currently, the craft show entrance fees cost Diamond $1,500 per year. The craft...
-
Use the logic diagram of Figure 27.2 to determine the point group for the planar molecule cisHBrC ¡ CClH. Indicate your decision-making process as was done in the text for NH 3 . a. linear? b....
-
Decompose the following reducible representation into irreducible representations of the C 2v group: 4
-
Show that z is a basis for the A 1 representation and that R Z is a basis for the A 2 representation of the C 3v group.
-
Hadley provides Ms. Kim with a Lexus that was purchased in 2022 for $58,000. In 2023, she drove 92,000 kilometres, with 85,000 for employment purposes and 7,000 for personal use. In 2023 Ms. Kim paid...
-
The data in the Table II were gathered using a force meter pulling horizontally on a block of wood placed on a level flat surface, but for the second part of this experiment the surface was not...
-
Use the given trigonometric ratio, which is based on actual side length measures, to sketch a right triangle and solve the triangle. 1 sin (A) Solution: Sketch:

Study smarter with the SolutionInn App


