The decomposition of mercury(II) thiocyanate produces an odd brown snake-like mass that is so unusual the process
Question:
The decomposition of mercury(II) thiocyanate produces an odd brown snake-like mass that is so unusual the process was once used in fireworks displays. There are actually several reactions that take place when the solid Hg(SCN)2 is ignited:
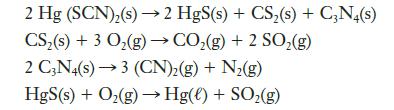
A 42.4-g sample of Hg (SCN)2 is placed into a 2.4-L vessel at 21°C. The vessel also contains air at a pressure of 758 torr. The container is sealed, and the mixture is ignited, causing the reaction sequence above to occur. Once the reaction is complete, the container is cooled back to the original temperature of 21°C.
(a) Without doing numerical calculations, predict whether the final pressure in the vessel will be greater than, less than, or equal to the initial pressure. Explain your answer.
(b) Calculate the final pressure and compare your result with your prediction. (Assume that the mole fraction of O2 in air is 0.21.)
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





