The decomposition of N 2 O 5 is given by the equation: The following mechanism is proposed
Question:
The decomposition of N2O5 is given by the equation:
![]()
The following mechanism is proposed for this reaction:
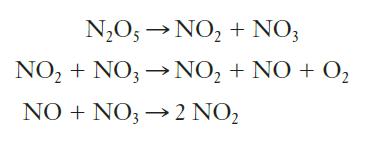
(a) Does this mechanism as written provide the correct stoichiometry? If not, how does it need adjustment?
(b) Identify all intermediates in the mechanism.
(c) Identify the molecularity of each step in the mechanism.
Strategy Consider the definitions for reaction mechanism and see how this example of a mechanism illustrates those definitions.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





