The Draco thrusters on SpaceXs Dragon spacecraft are based on the following reaction between monomethylhydrazine (CH 6
Question:
The Draco thrusters on SpaceX’s Dragon spacecraft are based on the following reaction between monomethylhydrazine (CH6N2) and dinitrogen tetroxide (N2O4):
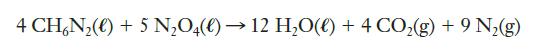
If either reactant is in excess, unnecessary mass will be added to the craft, so a stoichiometric mixture is desired. What mass of each reactant should be used for every kilogram of the fuel mixture?
Strategy We want to ensure that there will be no excess of either reactant. Because we are asked for the composition “for every kilogram” of fuel, we might start by setting the total mass of fuel to 1000 g. We can write that as an equation with two unknowns:
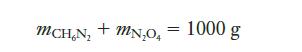
Because we have two unknowns, we will need a second relationship between the two masses. The balanced chemical equation shows us that the mole ratio between the two reactants should be 4:5. We can use molar masses to convert that to a ratio in terms of masses and then use that ratio as a second equation to solve the problem.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





