The rate of decomposition of SO 2 Cl 2 according to the reaction: can be followed by
Question:
The rate of decomposition of SO2Cl2 according to the reaction:
![]()
can be followed by monitoring the total pressure in the reaction vessel. Consider the following data:
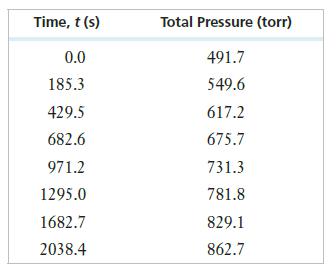
What must you do to convert these total pressures into changes in the pressure of the SO2Cl2? Manipulate the data as needed and then use a graphing calculator or spreadsheet to plot the data and determine the order of reaction and the rate constant.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





