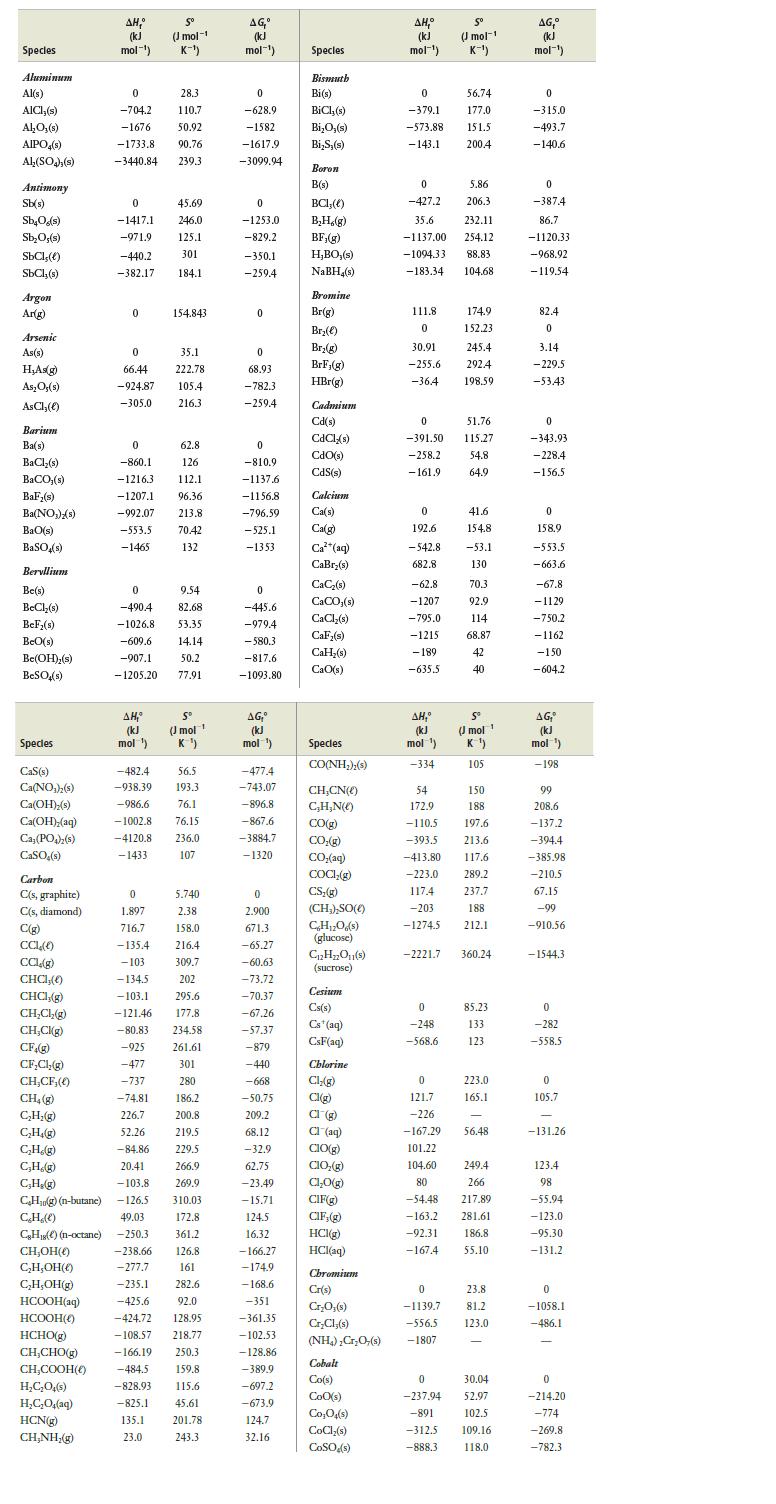
Use values from Appendix E at the back of the book to calculate the equilibrium constant, Kp,
Question:
Use values from Appendix E at the back of the book to calculate the equilibrium constant, Kp, for the following gaseous reactions:
(a) H2(g) + Cl2(g) ⇄ 2 HCl(g)
(b) CH4(g) + H2O(g) ⇄ CO(g) + 3 H2(g)
(c) SO2(g) + Cl2(g) ⇄ SO2Cl2(g)
(d) 2 HCl(g) + F2(g) ⇄ 2 HF(g) + Cl2(g)
Data from appendix E
Transcribed Image Text:
Specles Aluminum Al(s) AICI;(s) Al₂O₂ (s) AIPO4(s) Al₂(SO4),(s) Antimony Sb(s) Sb₂O(s) Sb₂O,(s) SbCl,() SbCl,(s) Argon Ar(g) Arsenic As(s) H₂As(g) As₂Os(s) AsCl₂(e) Barium Ba(s) BaCl₂(s) BaCO,(s) BaF,(s) Ba(NO₂)(s) BaD(s) BaSO4(s) Beryllium Be(s) BeCl₂(s) BeF₂(s) BeO(s) Be(OH)₂(s) BeSO4(s) Species Cas(s) Ca(NO₂)2(s) Ca(OH)₂(s) Ca(OH)₂(aq) Ca,(PO)(s) CaSO4(s) Carbon C(s, graphite) C(s, diamond) C(g) CCL(0) CCL,(g) CHCI,(Ⓒ) CHCI,(g) CH₂Cl₂(g) CH,CI(g) CF.(g) CF₂Cl₂(g) CH,CF,(0) CH₂(g) C₂H₂(g) C₂H₂(g) C₂H₂(g) C₂H₂(g) C₂H₂(g) C₂H₂(g) (n-butane) C₂H₂(e) CH₂OH() C₂H₂OH(Ⓒ) C₂H₂OH(g) HCOOH(aq) HCOOH(0) HCHO(g) CH,CHO(g) CH₂COOH() H₂C₂O4(s) H₂C₂O4(aq) AH, (kJ mol-¹) HCN(g) CH,NH,(g) 0 28.3 -704.2 110.7 -1676 50.92 -1733.8 90.76 -3440.84 239.3 0 -1417.1 -971.9 -440.2 -382.17 184.1 0 0 66.44 -924.87 -305.0 5⁰ (J mol-1 K-¹) 49.03 C,H₁(C)(n-octane) -250.3 45.69 246.0 ΔΗ, (kJ mol ¹) 125.1 301 154.843 0 62.8 -860.1 126 -1216.3 112.1 -1207.1 96.36 -992.07 213.8 -553.5 70.42 -1465 132 35.1 222.78 105.4 216.3 9.54 0 -490.4 82.68 -1026.8 53.35 -609.6 14.14 -907.1 50.2 -1205.20 77.91 Sº (mol 1 K¹) -482.4 56.5 -938.39 193.3 -986.6 76.1 -1002.8 76.15 -4120.8 236.0 -1433 107 0 5.740 1.897 2.38 716.7 158.0 -135.4 216.4 -103 309,7 -134.5 202 -103.1 295.6 -121.46 177.8 -80.83 234.58 -925 261.61 -477 301 -737 280 -74.81 186.2 226.7 200.8 52.26 219.5 -84.86 229.5 20.41 266.9 -103.8 269.9 -126.5 310.03 172.8 361.2 -238.66 126.8 -277.7 161 -235.1 282.6 -425.6 92.0 -424.72 128.95 -108.57 218.77 -166.19 250.3 -484.5 159.8 -828.93 115.6 -825.1 45.61 135.1 23.0 201.78 243.3 AG (kJ mol-¹) 0 -628.9 -1582 -1617.9 -3099.94 0 -1253.0 -829.2 -350.1 -259.4 0 0 68.93 -782.3 -259.4 0 -810.9 -1137.6 -1156.8 -796.59 -525.1 -1353 0 -445.6 -979.4 -580.3 -817.6 -1093.80 AG,⁰ (kJ mol ¹) -477.4 -743.07 -896.8 -867.6 -3884.7 -1320 0 2.900 671.3 -65.27 -60.63 -73.72 -70.37 -67.26 -57.37 -879 -440 -668 -50.75 209.2 68.12 -32.9 62.75 -23.49 -15.71 124.5 16.32 -166.27 -174.9 -168.6 -351 -361.35 -102.53 -128.86 -389.9 -697.2 -673.9 124.7 32.16 Specles Bismuth Bi(s) BiCl(s) Bi₂O,(s) Bi₂S,(s) Boron B(s) BCI,() B₂H,(g) BF,(g) HBO,(s) NaBH4(s) Bromine Br(g) Br₂(e) Br₂(g) BrF, (g) HBr(g) Cadmium Cd(s) CdCl(s) CdO(s) CdS(s) Calcium Ca(s) Ca(g) Ca²+ (aq) CaBr₂(s) CaC₂(s) CaCO₂ (s) CaCl₂(s) CaF₂(s) CaH₂(s) CaO(s) Specles CO(NH,),(s) CH₂CN() C₂H,N() CO(g) CO₂(g) CO,(aq) COCI,(g) CS₂(g) (CH₂) SO() C₂H₁₂O(s) (glucose) C₁₂H₂2O₁1(s) (sucrose) Cesium Cs(s) Cs¹ (aq) CsF(aq) Chlorine Cl₂(g) Cl(g) 1 (g) Cl(aq) CIO(g) CIO₂(g) Cl₂O(g) CIF(g) CIF; (g) HCl(g) HCl(aq) Chromium Cr(s) Cr₂O;(s) CryCl,(s) (NH4)₂Cr₂O,(s) Cobalt Co(s) Coo(s) Co₂O₂ (s) CoCl₂(s) COSO (s) AH,Ⓡ (kJ mol-¹) 0 -379.1 -573.88 -143.1 0 -427.2 111.8 0 30.91 -255.6 -36.4 35.6 232.11 -1137.00 254.12 -1094.33 88.83 -183.34 104.68 0 192.6 -542.8 682.8 -62.8 -1207 -795.0 -1215 -189 -635.5 5⁰ (mol-1 K-¹) AH, (kJ mol ¹) -334 56.74 177.0 151.5 200.4 0 51.76 -391.50 115.27 -258.2 - 161.9 5.86 206.3 0 -248 -568.6 174.9 152.23 245.4 292.4 198,59 54.8 64.9 41.6 154.8 -53.1 130 70.3 92.9 114 68.87 42 40 Sº (J mol 1 K¹) 105 54 150 172.9 188 -110.5 197.6 -393.5 213.6 -413.80 117.6 -223.0 289.2 117.4 237.7 -203 188 -1274.5 212.1 -2221.7 360.24 85.23 133 123 0 121.7 -226 -167.29 56.48 101.22 104.60 80 -54.48 -163.2 281.61 -92.31 186.8 -167.4 55.10 223.0 165.1 249.4 266 217.89 0 23.8 -1139,7 81.2 -556.5 123.0 -1807 0 30.04 -237.94 52.97 -891 102.5 -312.5 -888.3 109.16 118.0 AG, (kJ mol-¹) 0 -315.0 -493.7 -140.6 0 -387.4 86.7 -1120.33 -968.92 -119.54 82.4 0 3.14 -229.5 -53.43 0 -343.93 -228.4 -156.5 0 158.9 -553.5 -663.6 -67.8 -1129 -750.2 -1162 -150 -604.2 AG, (kJ mol ¹) -198 99 208.6 -137.2 -394.4 -385.98 -210.5 67.15 -99 -910.56 - 1544.3 0 -282 -558.5 0 105.7 -131.26 123.4 98 -55.94 -123.0 -95.30 -131.2 0 -1058.1 -486.1 0 -214.20 -774 -269.8 -782.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
Answer and explanation is attached below hope you like ...View the full answer

Answered By

Vijesh J
My passion to become a tutor is a lifetime milestone. Being a finance and marketing professional with hands-on experience in wealth management, portfolio management, team handling and actively contributing in promoting the company. Highly talented in managing and educating students in most attractive ways were students get involved. I will always give perfection to my works. Time is the most important for the works and I provide every answer on time without a delay. I will proofread each and every work and will deliver a with more perfection.
4.70+
5+ Reviews
15+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Calculate the standard free-energy change and the equilibrium constant Kp for the following reaction at 25oC. See Appendix C for data. CO(g) + 2H2(g) =CH,OH(g)
-
In a particular experiment, the equilibrium constant measured for the reaction, Cl 2 (g) + NO 2 (g) Cl 2 NO 2 (g), is 2.8. (a) Based on this measurement, calculate Go for this reaction. (b)...
-
The phase diagram for SO2 is shown here. (a) What does this diagram tell you about the enthalpy change in the reaction SO2(I) SO2(g)? (b) Calculate the equilibrium constant for this reaction at 100...
-
Last time i posted this someone solved with the wrong answer You are trying to calculate how much money you should have at retirement. On your 65 th birthday you will retire and immediately make your...
-
Because business writing should have high skim value, why not write everything in bulleted lists?
-
The Midwest Division of the Paibec Corporation manufactures subassemblies that are used in the corporations final products, Lynn Hardt of Midwests Profit Planning Department has been assigned the...
-
James Lewis, a resident of Kentucky, sustained an injury while operating a Caterpillar bulldozer. He filed suit against Caterpillar, a company incorporated in Delaware but with its principal place of...
-
Soldner Health Care Products Inc. expects to maintain the same inventories at the end of 2010 as at the beginning of the year. The total of all production costs for the year is therefore assumed to...
-
Explain data definition language (DDL). How is it different from data manipulation language (DML)?
-
Calculate the pH of a 0.10 M solution of propanoic acid and determine its percent ionization.
-
Write chemical equations and equilibrium expressions for the reactions of each of the following weak bases with water. (a) Ammonia, NH 3 , (b) Methylamine, CH 3 NH 2 , (c) Acetate ion, CH 3 COO ,...
-
Describe some generic types of record keys in typical accounting databases. Are such keys simple or complicated?
-
A PAPER OF ALOT OF PAGES ABOUT BARGAINING NEGOTIATION WITH A CULTURAL PERSPECTIVE AND CITE ALL REFERENCES PLEASE A brief introduction that discusses the organizational communication process and the...
-
In reviewing the team performance, considerations must be given to the following: How did your team come together and what phases or stages did it go through? What roles did team members play? o...
-
Show that the function f(x)=x 4 +5x+2 has exactly one zero in the interval [1,0].
-
A company purchases a machine for 1 0 0 , 0 0 0 on 1 January 2 0 X 5 . The machine has an estimated useful life of 1 0 years. On 1 January 2 0 X 8 the company enhances the machine by adding...
-
Aeglow Engineering, a contractor was awarded a $100 million contract to build a water treatment plant in Singapore. The Public Sector Standard Conditions of Contract (PSSCOC) for construction works...
-
Because real capital is supposed to earn a higher return where it is scarce, how do you explain the fact that most international investment flows to the IACs (where capital is relatively abundant)...
-
What is the shape of the exponential distribution?
-
If the beam is subjected to a moment of M = 100 kN m, determine the bending stress at points A, B, and C. Sketch the bending stress distribution on the cross section. 300 mm 30 mm 30 mm 150 mm 150 mm
-
If the beam is made of material having an allowable tensile and compressive stress of (Ï allow ) t = 125 MPa and (Ï allow ) c = 150 MPa, respectively, determine the maximum moment M that...
-
The shaft is supported by a smooth thrust bearing at A and smooth journal bearing at C. If d = 3 in., determine the absolute maximum bending stress in the shaft. -3 ft -3 ft -3 ft- 1800 lb 3600 Ib
-
Papst Company is preparing its cash budget for the month of May. The following information is available concerning its accounts receivable (based on sales made to customers on open account): Actual...
-
- = 3. For the Au Sn phase diagram given below draw schematics of plausible molar free energy curves showing the common tangent construction as a function of composition, xsn, at T 350C (30 at.% <...
-
analyze the: - Cost of Equity - Cost of Debt - Cost of Capital 2Book vs Market Value of the company. Does $Rl rely on debt? 3. Internal vs External Equity and Debt Due 4. Financing choices - Talk...

Study smarter with the SolutionInn App


