Using values from the table of standard reduction potentials, calculate the cell potentials of the following cells.
Question:
Using values from the table of standard reduction potentials, calculate the cell potentials of the following cells.
(a) ![]()
(b) 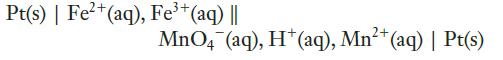
(c) ![]()
Transcribed Image Text:
Fe(s) | Fe²+ (aq) || Hg2+ (aq) | Hg()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To calculate the cell potentials of the given cells using the standard reduction potentials we will ...View the full answer

Answered By

Felix Onchweri
I have enough knowledge to handle different assignments and projects in the computing world. Besides, I can handle essays in different fields such as business and history. I can also handle both short and long research issues as per the requirements of the client. I believe in early delivery of orders so that the client has enough time to go through the work before submitting it. Am indeed the best option that any client that can think about.
4.50+
5+ Reviews
19+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Determine A in the indicated figures. Fig. 2.40 (c) B 4 66 A (c) 4 C
-
Using values from the table of standard reduction potentials, calculate the cell potentials of the following cells. (a) (b) (c) Ga(s) | Ga+ (aq) || Ag+ (aq) | Ag(s)
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) 3 Cu(s) + 2NO3(aq) + 8 H+ (aq) 3 Cu+ (aq) + 2 NO(g) +...
-
Data for Sabanci Holding can be found in the table below. Theincome statement items correspond to revenues or costs during theyear ending in either 2018 or 2019. The balance sheet itemscorrespond t 2...
-
List six general techniques that are effective in persuasion.
-
Design Arts Associates is an interior decorating firm in Berlin. The following costs were incurred in the firms contract to redecorate the mayors offices. Direct material used...
-
An effective way to learn how companies respond to the competing pressures to be globally integrated and locally responsive is to study them in action. Referring back to Exhibit 6.3, search online...
-
Every home football game for the past eight years at Eastern State University has been sold out. The revenues from ticket sales are significant, but the sale of food, beverages, and souvenirs has...
-
A child bounces a 48 g superball on the side- walk. The velocity change of the superball is from 23 m/s downward to 13 m/s upward. If the contact time with the sidewalk is 1 800 s, what is the...
-
Use the Nernst equation to calculate the cell potentials of the following cells at 298 K. (a) 2 Ag+ (aq)(0.50 M) + Ni(s)2 Ag(s) + Ni+ (aq)(0.20 M) (b) Cu(s) + PtCl (aq) (0.10 M) Cu+ (aq) (0.20 M)...
-
Bank 1 and Bank 2 are considering entering a compatibility agreement that would permit the users of each banks ATMs access to the other banks ATMs. Bank 1 has a network of branches and automated...
-
On January 4, 2017, Martin Corporation acquires two properties from a shareholder solely in exchange for stock in a transaction that qualifies under § 351. The shareholder's basis, the fair...
-
#include int *ptr; int arr1 [10] = {1, 2, 3, 4, 5, 6, 7, 8, 9,10}; int arr2 [10] = {11, 12, 13, 14, 15, 16, 17, 18, 19, 20}; int main() { } ptrarrl; printf("%d ", * (ptr+1)); ptrarr2; printf("%d ", *...
-
Find the present value of $30,000 due in 7 years at the given rate of interest. (Use a 365-day year. Round your answer to the nearest cent.) 3%/year compounded quarterly tA
-
1. A ladder 7 m long is leaning against a wall. If the bottom of the ladder is pushed horizontally toward the wall at 1.5 m/sec, how fast is the top of the ladder sliding up the wall when the bottom...
-
(9%) Problem 11: Two ice skaters, each with the same mass, m, skate in opposite directions at the same speed of v. For the purposes of this problem, treat each skater as a point mass. Their...
-
For each of the following resident individual taxpayers, calculate the amount that they would be entitled to claim under either s.US-6Oor s.25-65 as a deduction for election expenses forthe2O2J/22...
-
Use the true values x1 = 7 and x2 = 8. (a) Demonstrate the correctness of the error equation from Prob. 110 for addition if three correct digits are used for X1 and X2. (b) Demonstrate the...
-
Rewrite Programming Exercise 7.5 using streams. Display the numbers in increasing order. Data from Programming Exercise 7.5 Write a program that reads in 10 numbers and displays the number of...
-
Determine the maximum normal stress developed in the bar when it is subjected to a tension of P = 2 kip. 0.125 in. 1.875 in. 1.25 in. r = 0.25 in. 0.75 in.
-
Determine the maximum axial force P that can be applied to the bar. The bar is made from steel and has an allowable stress of Ï allow = 21 ksi. 0.125 in. 1.875 in. 1.25 in. r = 0.25 in. 0.75 in.
-
The A-36 steel plate has a thickness of 12 mm. If Ï allow = 150 MPa, determine the maximum axial load P that it can support. Calculate its elongation, neglecting the effect of the fillets. r= 30...
-
Document A We hold that the policy known as imperialism is hostile to liberty and tends toward militarism, an evil from which it has been our glory to be free. We regret that it has become necessary...
-
The hectare yields approximately 9000 kg of corn. The corn is then processed in an ethanol plant to make almost pure (99.5%) ethanol. Per 1000 liters of ethanol, the ethanol production process...
-
2. You are tasked to use a strong-acid polystyrene cross-linked with pure divinyl benzene ion- exchanger resin operated with a sulfonated sodium to remove aluminum from a wastewater stream. The...

Study smarter with the SolutionInn App


