When hydrogen gas reacts with iodine gas at elevated temperatures, the following equilibrium is established: A student
Question:
When hydrogen gas reacts with iodine gas at elevated temperatures, the following equilibrium is established:
![]()
A student measured the equilibrium constant as 59.3 at 4008C. If one trial begins with a mixture that includes 0.050 M hydrogen and 0.050 M iodine, what will be the equilibrium concentrations of reactants and products?
Strategy
It may help to begin by summarizing the information from the problem.
Given: [H2]initial = [I2]initial = 0.050 M; K = 59.3 Find: [H2]eq, [I2]eq, and [HI]eq
We’ll use (and clarify) the three-point strategy outlined above. The chemical equation for the equilibrium is given in the problem. From that equation, we can write the equilibrium expression easily enough:
![K = [HI] [H] [1] 59.3](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/7/8/4316564659fed3d51701078431726.jpg)
Next, we construct the table of concentrations, with places to enter the initial concentrations, the changes in concentration, and the final (equilibrium) concentrations for the three species. So far, only the given initial concentrations are known. (Because only H2 and I2 are present initially, the initial concentration of HI is zero.)
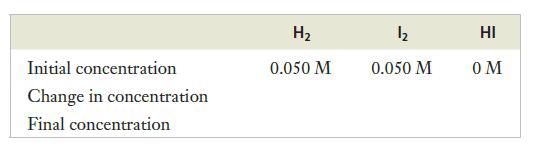
The rest of the table will be filled in as we work our way through the solution. At this point, the problem seems daunting; we have three unknown equilibrium concentrations, and only one real equation to work with—the equilibrium expression. We will need to use the stoichiometry of the reaction to relate the changes in concentration to one another.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





