100.0 J of heat is added to a 3.45-g sample of an unknown metal. The temperature of...
Question:
100.0 J of heat is added to a 3.45-g sample of an unknown metal. The temperature of the metal increases from 22.37 °C to 54.58 °C. Use the date in Table 5.1 to identify the metal.
Table 5.1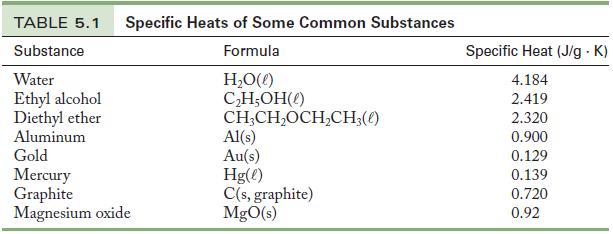
Transcribed Image Text:
TABLE 5.1 Specific Heats of Some Common Substances Substance Formula H₂O(l) C₂H₂OH() CH₂CH₂OCH₂CH3(0) Water Ethyl alcohol Diethyl ether Aluminum Gold Mercury Graphite Magnesium oxide Al(s) Au(s) Hg(e) C(s, graphite) MgO(s) Specific Heat (J/g.K) 4.184 2.419 2.320 0.900 0.129 0.139 0.720 0.92
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
AH100 J mass g345g AT TT5458 C2237 ...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
1. Examine the hanging drop slide and complete the following table with respect to the size, shape, and motility of the different bacteria. Bacterium B subtilis P aeruginosa S volutans Size 2. Draw a...
-
A shareholder made a loan of 1,000 to a corporation in which she owned 50% of the stock. The loan is evidenced by a promissory note with the word "LOAN" printed at the top. The loan has a market...
-
You work for a gas turbine design company and have a client who has a fairly loose specification for a gas turbine engine. You are required to design an aviation gas turbine to power the aircraft...
-
Repeat parts (g) and (i) of Example 17.9 if the solution velocity past the crystal face is reduced from 5 cm/s to 1 cm/s.
-
a. Identify the functions local extreme values in the given domain, and say where they occur. b. Which of the extreme values, if any, are absolute? c. Support your findings with a graphing calculator...
-
Selected accounts from the trial balance as at 30 June 2026 of the partnership of Delta, Alpha and Omega are as follows. End-of-period adjustments for the financial year ended 30 June 2026 have yet...
-
Roman Products, Inc., is a wholesaler of mens hair products. The company began operations on January 1, 2010. The following transactions relate to securities acquired by Roman Products, Inc., which...
-
5 Which one of the following correctly represents Sodium oxide? +2 XX a) Na 20 XX 6) 2NaOx x; 1-2 'xx' 1-2 c) 2 Na 20% c) Na XX XX 6 An element with atomic number_ will form a basic oxide. a) 7 (2,5)...
-
Sherry Tsang has just started up a small corporation that produces clothing. She has applied for and received a government grant. The grant will automatically be renewed as long as the business shows...
-
It takes 677 J of heat to increase the temperature of 25.0 g liquid ethanol (C 2 H 5 OH) from 23.5 C to 34.7 C. What is the specific heat of this substance?
-
What mass of acetylene, C 2 H 2 (g), must be burned to produce 3420 kJ of heat, given that its enthalpy of combustion is -1301 kJ/mol? Compare this with the answer to Exercise 5.91 and determine...
-
Find an equation of the tangent line to the curve at the given point. y = sin x + sin 2 x, (0,0)
-
The parameter to the following two recursive routines is a pointer to a singly linked list of numbers, whose elements are unique (no duplicates) and unsorted. Each node in the list contains two...
-
Prepare a budget for a family of four with an annual income of \(\$ 250,000\).
-
Write down the Walsh matrix for \(m=4\).
-
In Problems 21-38, guess the requested limits. \(\lim _{n ightarrow \infty} \frac{1-n}{n+10}\)
-
Two packs \(\mathrm{A}\) and \(\mathrm{B}\) of mass \(m_{A}=2.2 \mathrm{~kg}\) and \(m_{B}=2.8 \mathrm{~kg}\) are connected by an inextensible rope of negligible mass. Pack A rests on an inclined...
-
Consider projects Alpha and Beta: The opportunity cost of capital is 8%. Suppose you can undertake Alpha or Beta, but not both. Use the IRR rule to make the choice. (Whats the incremental investment...
-
a. What is meant by the term tax haven? b. What are the desired characteristics for a country if it expects to be used as a tax haven? c. What are the advantages leading an MNE to use a tax haven...
-
At 350. K, pure toluene and hexane have vapor pressures of 3.57 10 4 Pa and 1.30 10 5 Pa, respectively. a. Calculate the mole fraction of hexane in the liquid mixture that boils at 350. K at a...
-
The partial molar volumes of water and ethanol in a solution with xH 2 O = 0.45 at 25C are 17.0 and 57.5 cm 3 mol 1 , respectively. Calculate the volume change upon mixing sufficient ethanol with...
-
A solution is made up of 222.9 g of ethanol and 130.8 g of H 2 O. If the volume of the solution is 403.4 cm 3 and the partial molar volume of H 2 O is 17.0 cm 3 mol 1 , what is the partial molar...
-
What is the difference between operating budgets and financial statements?
-
what clause states weather the government or the contractor is generally responsible for shipping costs and assumes the risk of loss for supplies prior to and during transit?
-
What are the key features of organisational policies and procedures in reference to Financial Administration and Budgeting?

Study smarter with the SolutionInn App


