Although copper does not usually react with acids, it does react with concentrated nitric acid. The reaction
Question:
Although copper does not usually react with acids, it does react with concentrated nitric acid. The reaction is complicated, but one outcome is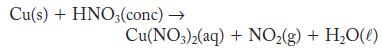
(a) Name all of the reactants and products.
(b) Balance the reaction.
(c) Assign oxidation numbers to the atoms. Is this a redox reaction?
(d) Pre-1983 pennies were made of pure copper. If such a penny had a mass of 3.10 g, how many moles of Cu are in one penny? How many atoms of copper are in one penny?
(e) What mass of HNO3 would be needed to completely react with a pre-1983 penny?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





