Determine the molarity of an aqueous solution that is 37.2% in HCl. The density of this solution
Question:
Determine the molarity of an aqueous solution that is 37.2% in HCl. The density of this solution is 1.034 g/mL.
Strategy
Write the concentrations as fractions.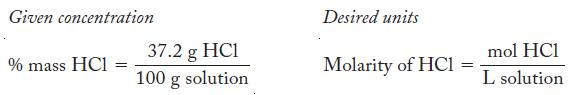
The units in the numerator of percentage composition can be easily converted to moles, and we can use the density of the solution to convert from grams of solution to volume of solution.
Transcribed Image Text:
Given concentration % mass HC1 = 37.2 g HC1 100 g solution Desired units Molarity of HC1 = mol HCl L solution
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The molar mass of HCl is 3646 gmol Converting the a...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
I Jose and Laura go to a rock concert. Jose doesn't feel well and wants to go home, but Laura cannot hear Jose over the music. What component of communication are Jose and Laura experiencing? O...
-
Consider a 27-year bond with $1,000 face value that pays a 8.00% coupon on an annual basis and has a yield-to-maturity of 7.00%. What is the approximate percentage change in the price of bond if...
-
Alleghany Community College operates four departments. The square footage used by each department is shown below. Alleghany's annual building rental cost is $320,000 What amount of rent expense that...
-
Find the impedance ZL for maximum average power transfer and the value of the maximum average power transferred to ZL for the circuit shown in figure. 12/0 V j1n 310 10 > 21 ZL -j1n
-
You are working on a special assignment as a financial analyst for the president of household products of RBB Brands. RBB Brands is a large $ 4 billion diversified consumer products firm. RBB has two...
-
Describe the wide variety of legal issues that occur in the emergency department setting.
-
SummerFun, Inc., produces a variety of recreation and leisure products. The production manager has developed an aggregate forecast: Develop an aggregate plan using each of the following guidelines...
-
3. Let R be the relation on the set {1,2,3,4) containing the ordered pairs (1,3), (1,4), (2,1), (2,2), (2,3), (3,1), (3,4), and (4,3). a. (10 pts) Draw the directed graph representing the relation R....
-
Predict the solvent in which the given compound is more soluble, and justify your prediction. Strategy Consider the types of intermolecular interactions in the solute and the solvents. Solubility...
-
Find the molarity of a 7.85% aqueous ammonia solution that has a density of 0.965 g/mL.
-
Vertical analysis (common size) percentages for Dagman Company's sales revenue, cost of goods sold, and expenses are shown below. Did Dagman's net income as a percentage of sales increase, decrease,...
-
You are vice president, international business development, for lines 'R Us, a manufacture of production equipment. there is a big demand for your equipment in china and you are looking to set up...
-
When building a new hospitality facility, managers need to build a capacity to accommodate the highest customer demand in order to satisfy customers at all time. Explain
-
One month from today your company will transfer you to live in a foreign country different from your own to work in their overseas office. What are the types of preparations for adapting to a foreign...
-
We are now living in a new era of technology. Businesses have been declining due to their inability to adapt to this new technological era. Take a look around Belize City and share with us Businesses...
-
What are the root causes of cyber security in the healthcare system during covid 19? Analysis of phishing, ransomware, distributed denial of service (DDoS) attacks, and malware. Cite and provide...
-
Refer back to Chapter, in particular to the Battle of the Sexes in Table. Imagine that these payoffs are monetary payoffs. a. Suppose that players only care about monetary payoffs, with $1 = 1 utile....
-
Which of the ocean zones shown would be home to each of the following organisms: lobster, coral, mussel, porpoise, and dragonfish? For those organisms you identify as living in the pelagic...
-
Determine the displacement at D and the slope at D. Assume A is a fixed support,Bis a pin, and C is a roller. Use the conjugate-beam method. |B Ic |A 12 ft 12 ft 12 ft
-
Determine the displacement at D and the slope at D. Assume A is a fixed support,Bis a pin, and C is a roller. Use the moment-area theorems. 6 k D B |A - 12 ft 12 ft 12 ft-
-
Determine the displacement at C. Assume A is a fixed support,Bis a pin, and Dis a roller. EI is constant. Use the conjugate-beam method. 25 kN -3 m -3 m 3 m
-
A five-year $100 par value step-up note pays coupons semi-annually and has the following coupon rate structure: the annual coupon rate is 4% in year 1, which then rises by 50 bps every year over the...
-
discuss this question: what do you perceive to be the purpose and role of Accounting? How is accounting information potentially relevant to you in your area of business? After posting your response,...
-
What are the different types of proprietary funds? Use your CAFR to discuss the proprietary funds used by your chosen government. Do any of these funds use exchange transactions? What financial...

Study smarter with the SolutionInn App


