Determine the rate law and rate constant for the decomposition of the organic compound 1,3-pentadiene. 1,3-pentadiene
Question:
Determine the rate law and rate constant for the decomposition of the organic compound 1,3-pentadiene.
1,3-pentadiene → products
The concentration of 1,3-pentadiene was measured as a function of time. The data are as follows:![Time (s) 0 1000 2000 3000 4000 5000 [1,3-pentadiene] (M) In[1,3-pentadiene] -0.734 -1.720 -2.207 -2.532](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/4/4/41665963b60b72ec1704344416307.jpg)
Strategy
A logic flow diagram shows the strategy. We will prepare up to three graphs: concentration versus time to check for zero-order kinetics, ln[concentration] versus time to check for first-order kinetics, and 1/[concentration] versus time to check for second-order kinetics.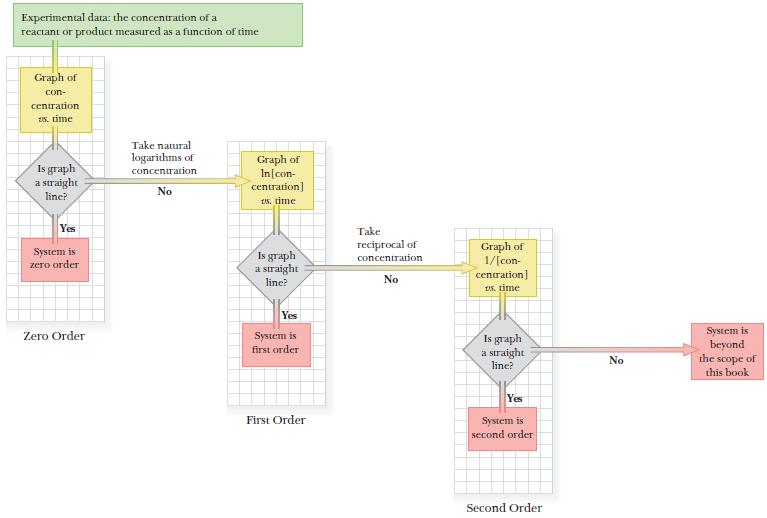
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





