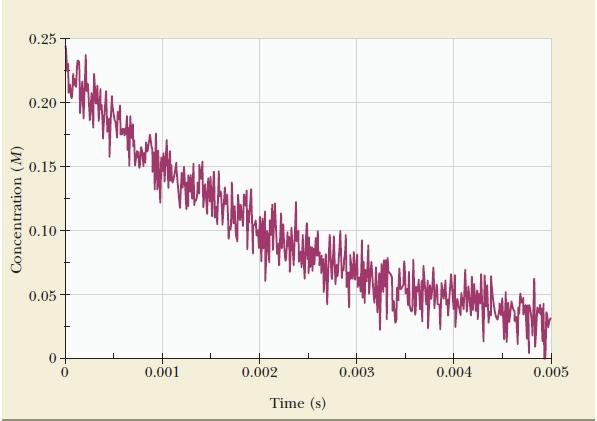
Determine the rate law (order and rate constant) from the data on the next page, obtained in
Question:
Determine the rate law (order and rate constant) from the data on the next page, obtained in a research laboratory that studies fast reactions. Assume that the reaction is![]()
Strategy
First, determine the rate law for the reaction using the strategy shown in the block diagram. Th e core of our strategy is to graph reactant concentration versus time and determine whether the relationship is a straight line. If not, graph ln[concentration] versus time and determine whether that relationship is a straight line.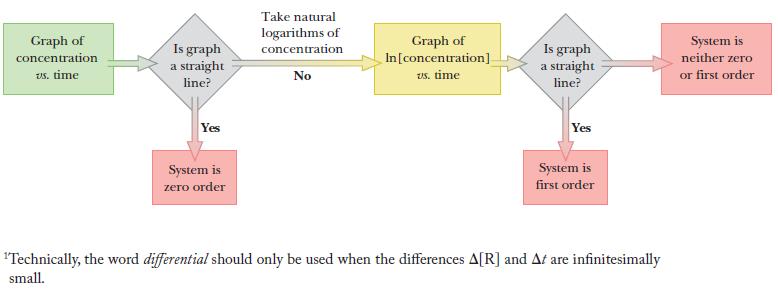
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





