Exactly 0.00200 mol hydrogen iodide is placed in a 5.00-L flask, and the temperature is increased to
Question:
Exactly 0.00200 mol hydrogen iodide is placed in a 5.00-L flask, and the temperature is increased to 600 K. Some of the HI decomposes, forming hydrogen and the violetcolored iodine gas:![]()
After the system reaches equilibrium, the concentration of iodine is determined by measuring the absorption of radiation (a quantitative measurement of the intensity of the purple color) and is found to be 3.8 × 10-5 M. Calculate Kc for this system at 600 K.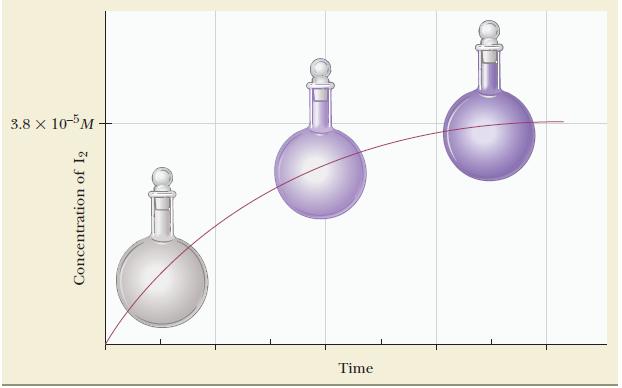
Strategy
Write the balanced equation, the iCe table, the algebraic expression for Kc, substitute values from the iCe table, and solve.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





