Give the notation (1s, 2s, 2p, and so on) for each of the following subshells. If the
Question:
Give the notation (1s, 2s, 2p, and so on) for each of the following subshells. If the combination is not allowed, state why.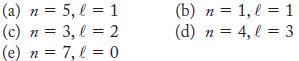
Transcribed Image Text:
(a) n = 5, l = 1 (c) n = 3, l = 2 (e) n = 7, l = 0 (b) n = 1, l=1 (d) n = 4, l = 3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The notation for subshells follows the format nl where n is the principal ...View the full answer

Answered By

Joseph Njoroge
I am a professional tutor with more than six years of experience. I have helped thousands of students to achieve their academic goals. My primary objectives as a tutor is to ensure that students do not have problems while tackling their academic problems.
4.90+
10+ Reviews
27+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Shown below is a qualitative diagram of the atomic orbital energies for an Na atom. The number of orbitals in each subshell is not shown. (a) Are all of the subshells for n = 1, n = 2, and n = 3...
-
Determine whether each of the following electron configurations represents the ground state or an excited state of the atom given. (a) C (c) Be 1s 2s 1s 2s 2p 2p (b) N 1s 2s (d) ON N 1s 2s 2p 2p
-
A cam is to provide follower motion as follows: The follower rises, with constant velocity, 4 inches in 2 seconds. The follower then dwells for 3 seconds. The follower then falls, 3 inches, with...
-
On 1 June 2019, Manchester United Ltd bought 48 million ordinary shares in Chelsea FC Ltd paying GHS 280 million cash. The summarised statement of financial position for the two entities as at 31...
-
A 4-kg mass is supported by a steel wire of diameter 0.6 mm and length 1.2 m. How much will this wire stretch under this load?
-
1. Would you characterize television programming decisions as structured or unstructured? Explain. What type of decision-making condition would you consider this to be? Explain. 2. What criteria did...
-
Consider the delivery time data in Example 3.1. In Section 4.2.5 noted that these observations were collected in four cities, San Diego, Boston, Austin, and Minneapolis. Example 3.1 a. Develop a...
-
The controller for Rainbow Childrens Hospital, located in Munich, Germany, estimates that the hospital uses 30 kilowatt-hours of electricity per patient-day, and that the electric rate will be .10...
-
The function g is defined by g(x, y)=3+x-x - y on the domain D given by points in the xy-plane satisfying x+ y1 and x0. (a) Find the stationary points of the function g, and classify them. (b) Find...
-
You are entering the widget business. It costs $500,000, payable in year 1, to develop a prototype. This cost can be depreciated on a straight-line basis during years 15. Each widget sells for $40...
-
Give the notation (1s, 2s, 2p, and so on) for each of the following subshells. If the combination is not allowed, state why. l=1 (a) n = 6, (c) n = 5, l = 2 (e) n = 2, l = 3 (b) n = 3,l = 0 (d) n =...
-
Give the values of the n and quantum numbers for the subshells identified by the following designations. (a) 3p (c) 7s (e) 2s (b) 5d (d) 4f 69
-
All marigolds are annuals. Some tulips are annuals. Some tulips are marigolds. Use an Euler diagram to determine whether the argument is valid or invalid.
-
Unroll the loop below: a. two times b. three times for (i = 0; i < 32; i++) x[i] = a[i] * c[i];
-
Einstein once said that Everything should be made as simple as possible, but not simpler. This perfectly captures the view that accounting standards should be firmly governed by high-level principles...
-
Look up the FASB home page on the Internet at the following address: www.fasb.org. Find the list of technical projects that are currently on the boards agenda. Choose one of the projects that will...
-
An elevator operated by an electric motor rises at a constant speed. What is the work done on the elevator as it rises a distance \(b\) ?
-
About how much work does a trash collector do in the course of an 8-hour shift?
-
A data firm records a large amount of data. Historically, .9% of the pages of data recorded by the firm contain errors. If 200 pages of data are randomly selected, a. What is the probability that six...
-
7 A 29-year-old, previously healthy man suddenly collapses at a party where legal and illicit drugs are being used. Enroute to the hospital, he requires resuscitation with defibrillation to establish...
-
Some symmetry operations can be carried out physically using a ball-and-stick model of a molecule without disassembly and reassembly and others can only be imagined. Give two examples of each...
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for 1,1,1,2-tetrachloroethane. Justify your answer. I H1 --c-CI CI | Cl
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for 1, 1, 2, 2-tetrachloroethane assuming that there is no rotation of the two...
-
Prove Ther Compute the kernel for the homomorphism : ZZ such that o(1) = 12. is {0}. Let ZZ and o(1) = 12. : Then ker() = {x Z\o(x) = 0}. Since (x)=(1+1+1+...x times) = x(1) and (1) = 12, then (x) =...
-
Diamond-Dybvig (1983) model of banking Equilibrium 1 Suppose everyone believes that only Type A people will withdraw at T=1. In this case the fraction of withdrawers is = 0. The Type A folks receive...
-
Find all subgroups of 72 x 7y of order 4 264 26.2 x 24. (0,0) (1,0) Answer: (0,1) (1,1) (0,2) (1,2) {(0,0), (0,1), (0,2), (0, 3)} and (0,3) (1,3) {(1, 0), (1, 1), (1,2), (1,3)} Subgroups of order 4

Study smarter with the SolutionInn App


