Hydrogenation of hydrocarbons is an important reaction in the chemical industry. A simple example is the hydrogenation
Question:
Hydrogenation of hydrocarbons is an important reaction in the chemical industry. A simple example is the hydrogenation of ethylene to form ethane. Calculate the enthalpy change for ![]()
Use the following thermochemical equations to determine the overall enthalpy change.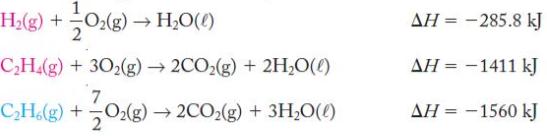
Strategy
Identify the position of the reactants (C2H4, H2) and products (C2H6) from the target equation in each of the individual thermochemical equations you wish to add. Reverse equations if necessary to put these species on the correct side and change the sign of ΔH accordingly. Compounds that do not appear in the target equation must cancel from the reactant and product sides. Remember that if you must multiply or divide an equation by a whole number, you must also perform the same operation on ΔH for that equation.
Step by Step Answer:

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball





