In each of the following pairs of bonds, which bond is more polar? Show the direction of
Question:
In each of the following pairs of bonds, which bond is more polar? Show the direction of the dipole moment for the more polar bond.
(a) H–C or H–N
(b) O–C or Cl–N
(c) S–O or S–F
Strategy
Use the table of electronegativities in Figure 9.9 to determine the electronegativity difference. The more polar bond will be the one with the larger difference in electronegativity. The arrow points toward the more electronegative element and it’s length is related to the electronegativity difference.
Figure 9.9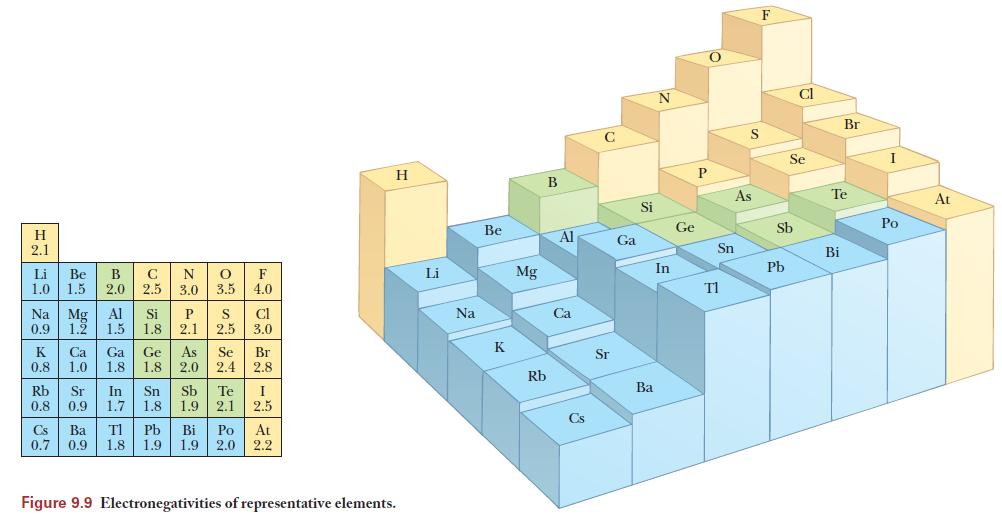
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





