State whether increasing temperature increases or decreases the value of the equilibrium constant for the following reactions.
Question:
State whether increasing temperature increases or decreases the value of the equilibrium constant for the following reactions.
For each reaction, an equilibrium constant at 298 K is given. Calculate ΔG ° for each reaction.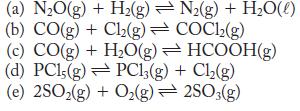
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a K eq decreases with increasing temperature ...View the full answer

Answered By

Krishnavendra Y
I am a self motivated financial professional knowledgeable in; preparation of financial reports, reconciling and managing accounts, maintaining cash flows, budgets, among other financial reports. I possess strong analytical skills with high attention to detail and accuracy. I am able to act quickly and effectively when dealing with challenging situations. I have the ability to form positive relationships with colleagues and I believe that team work is great key to performance. I always deliver quality, detailed, original (0% plagiarism), well-researched and critically analyzed papers.
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
State whether increasing temperature increases or decreases the value of the equilibrium constant for the following reactions. For each reaction, an equilibrium constant at 298 K is given. Calculate...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
(a) LEP Table 12-2: Exothermic Reaction with Heat Exchange Download the Polymath, MATLAB, Python, or Wolfram codes for the algorithm and data given in Table T12-2 for the exothermic gas phase...
-
2. A firm's or cash flow, is its profits after deductions for all expenses, including or wear and tear on capital goods such as machinery.
-
A sphere is suspended in air in a dark room and maintained at a uniform incandescent temperature. When first viewed with the naked eye, the sphere appears to be brighter around the rim. After several...
-
The following electrostatic potential diagrams represent CH4, NH3, or H2O. Label each, and explain your choices. a. C. b.
-
A capital investment project involves the purchase of a 3-D soldering station for $\$ 15,000$, producing an annual net benefit of $\$ 2,250$. The system has an 8-year useful life with a salvage value...
-
Table gives hypothetical export price indexes and import price indexes (1990 100) for Japan, Canada, and Ireland. Compute the commodity terms of trade for each country for the period 19902006. Which...
-
Beginning raw materials inventory $96,000 Beginning work-in-process inventory 168,000 Beginning finished goods inventory 600,000 Raw materials purchases 684,000 Wages paid 372,000 Applied...
-
Calculate the vapor pressure of each of the following at the given temperature. For each reaction, an equilibrium constant at 298 K is given. Calculate G for each reaction.
-
Calculate G and G at 37 C for the following equation. For each reaction, an equilibrium constant at 298 K is given. Calculate G for each reaction.
-
The Great Northwest Outdoor Company is a catalogue sales operation that specializes in outdoor recreational clothing. Demand for its items is very seasonal, peaking during the holiday season and...
-
The Eurocurrency market is the Question 19 options: a) medium-term portion of the Euromarket. b) short-term portion of the Euromarket. c) long-term portion of the Euromarket. d) None of the above.
-
What effect does a decrease in the total money supply have on the interest rate, investment amount, national income and the general level of prices in the short term? Explain by drawing a graph
-
Why would a company issuing new stock "provide an exit for its existing investors by issuing more shares of common stock and allowing them to sell their shares of stock to the new investors"?
-
Suppose in a Country Pinappleville, there are two socio-economic classes. The working class accounts for 90% of the population. Each person in the working class has a wealth of w, where w>0. The rest...
-
A big supplier of used cars is rental car companies, who sell their rental cars after they're too used to rent out but are still good. Rental cars are not a substitute or complement for used cars on...
-
What was the nature of the fraud at Equity Funding? How was the fraud covered up? What type of business analysis would have revealed the fraud?
-
Data 9.2 on page 540 introduces the dataset Cereal, which includes information on the number of grams of fiber in a serving for 30 different breakfast cereals. The cereals come from three different...
-
Find the volume flow rate of water exiting from the tank shown in Fig. 7.12. The tank is sealed with a pressure of 140 kPa above the water. There is an energy loss of 2.0 Nm / N as the water flows...
-
Water at 40°F is flowing downward through the fabricated reducer shown in Fig. 7.11. At point A the velocity is 10 ft/s and the pressure is 60 psig. The energy loss between points A and B is 25...
-
A horizontal pipe carries oil with a specific gravity of 0.83. If two pressure gages along the pipe read 74.6 psig and 62.2 psig, respectively, calculate the energy loss between the two gages.
-
Need help determining what normalization rules can be applied to my database. Attached is a copy of my database that I made in MySQL. Need to apply the Normalization rules to my database design. And...
-
The average age of promotional coupon holders of a clothing store was determined through a past research study and is 30 years old. In order to test whether the average age has increased in the...
-
Expressing empathy is crucial to meaningful communication, but it is not an easily acquired skill; it takes practice. Here are a few practice examples. For any one or two of the following situations,...

Study smarter with the SolutionInn App


