The electrochemical processes that occur in the corrosion of iron are represented by the half-reactions (a) Write
Question:
The electrochemical processes that occur in the corrosion of iron are represented by the half-reactions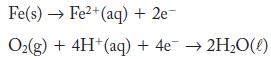
(a) Write the overall reaction.
(b) What is the standard potential for the overall chemical reaction?
(c) Natural water has a pH of about 5.9, and air is 0.21 mol fraction oxygen. If the concentration of iron(II)
in the water is 5 × 10-5 M, what is the potential of the corrosion reaction in the presence of air and natural water at 1 atm pressure and 298 K?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





