The nuclide 31Si is a radioactive isotope that disintegrates by beta decay. A sample of silicon-31 is
Question:
The nuclide 31Si is a radioactive isotope that disintegrates by beta decay.![]()
A sample of silicon-31 is found to produce 251 disintegrations/s . Exactly 3.00 hours later, the same sample produces 113 disintegrations/s . What is the half-life of 31Si?
Strategy The relative rate of decay is proportional to the number of atoms present, so we can use Equation 21.3 to calculate the half-life as follows:
Equation 21.3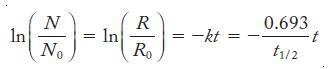
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





