The Understanding section in Example 9.10 showed two possible arrangements for the atoms in H 2 CO.
Question:
The Understanding section in Example 9.10 showed two possible arrangements for the atoms in H2CO. We concluded that structure A, repeated here, was better. A third possible structure follows as B. Use formal charge to predict which of these arrangements is the more favored.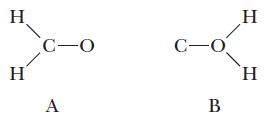
Example 9.10
There are two possible ways to connect the atoms in hydrogen cyanide: HCN and HNC. Write the Lewis structures and use formal charges to predict which is the more likely structural arrangement.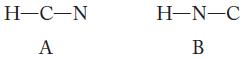
Strategy
Write the Lewis structure for each arrangement and evaluate the formal charges to predict the more likely structure.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





