Tin(II) fluoride (stannous fluoride) is added to toothpaste as a convenient source of fluoride ion, which is
Question:
Tin(II) fluoride (stannous fluoride) is added to toothpaste as a convenient source of fluoride ion, which is known to help minimize tooth decay. The concentration of stannous fluoride in a particular toothpaste can be determined by precipitating the fluoride as the mixed salt PbClF.
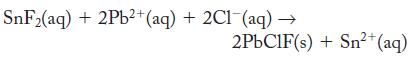
Th e concentrations of Pb2+ and Cl- are controlled so that PbCl2 does not precipitate. If a sample of toothpaste that weighs 10.50 g produces a PbClF precipitate that weighs 0.105 g, what is the mass percentage of SnF2 in the toothpaste?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





