Use the data in Figure 13.1 to calculate the instantaneous rate of disappearance of the reactant at
Question:
Use the data in Figure 13.1 to calculate the instantaneous rate of disappearance of the reactant at 10 seconds.
Strategy
The instantaneous rate is the slope of the tangent, so place a ruler on the graph, draw the tangent, and calculate the slope. Although two people might not place the ruler in exactly the same position, slopes estimated from graphs are surprisingly accurate.
Figure 13.1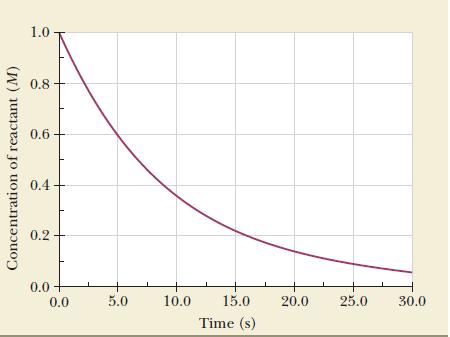
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





