Write the expressions for both K p and K c for the following reactions: Strategy Remember that
Question:
Write the expressions for both Kp and Kc for the following reactions: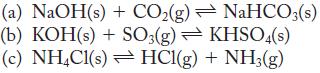
Strategy
Remember that pure solids and liquids do not appear in the expression for the equilibrium constant.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





