(a) With reference to Figure 6.19, what is the relationship between the number of nodes in an...
Question:
(a) With reference to Figure 6.19, what is the relationship between the number of nodes in an s orbital and the value of the principal quantum number?
Figure 6.19
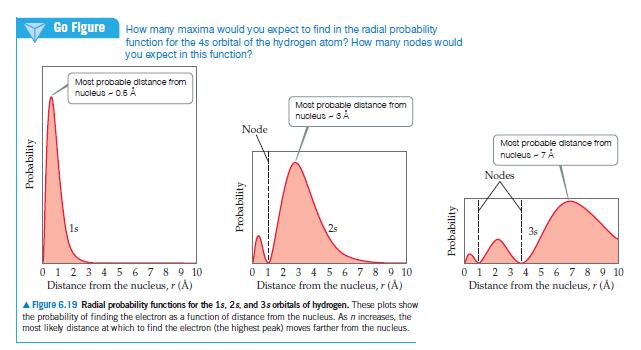
(b) Identify the number of nodes; that is, identify places where the electron density is zero, in the 2px orbital; in the 3s orbital.
(c) What information is obtained from the radial probability functions in Figure 6.19?
(d) For the hydrogen atom, list the following orbitals in order of increasing energy: 3s, 2s, 2p, 5s, 4d.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





