An ideal gas at a pressure of 1.50 atm is contained in a bulb of unknown volume.
Question:
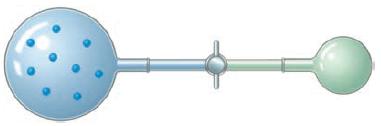
An ideal gas at a pressure of 1.50 atm is contained in a bulb of unknown volume. A stopcock is used to connect this bulb with a previously evacuated bulb that has a volume of 0.800L as shown here. When the stopcock is opened the gas expands into the empty bulb. If the temperature is held constant during this process and the final pressure is 695 torr, what is the volume of the bulb that was originally filled with gas?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:





