Compounds like sodium stearate, called surfactants in general, can form structures known as micelles in water, once
Question:
Compounds like sodium stearate, called “surfactants” in general, can form structures known as micelles in water, once the solution concentration reaches the value known as the critical micelle concentration (cmc). Micelles contain dozens to hundreds of molecules. The cmc depends on the substance, the solvent, and the temperature.
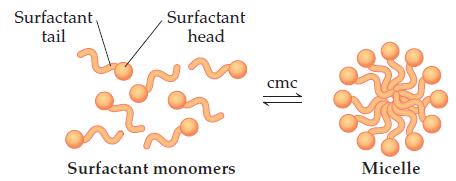
At and above the cmc, the properties of the solution vary drastically.
(a) The turbidity (the amount of light scattering) of solutions increases dramatically at the cmc. Suggest an explanation.
(b) The ionic conductivity of the solution dramatically changes at the cmc. Suggest an explanation.
Surfactant, tail Surfactant head cmc Surfactant monomers Micelle
Step by Step Answer:
a Critical micelles concentration is defined as concentration of surfactant above ...View the full answer



Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Related Video
Using a few basic physics principles, you can impress your friends with a trick that makes a bottle disappear. For this experiment, you will need a mini plastic bottle, glycerin, a glass, and a funnel. First, open the bottle and pour some glycerin into it, then close the bottle tightly. Next, pour some water into the glass using a funnel. Place the mini bottle into the glass of water and it will look normal. This is because light travels through air faster than it travels through the glass and water, allowing our eyes to see the bottle inside the glass. However, when you fill the mini bottle with more glycerin, and pour glycerin into the glass, then put the glycerin filled bottle into the glass with glycerin in it. Half of the bottle that is submerged in the glycerin will become invisible as the light travels through glass and glycerin at the same speed, thus it does not bend and no refraction takes place, making the bottle invisible. This happens because both glass and glycerin have almost the same refractive index, which causes the speed of light to be the same in both mediums, causing no bending of light and making the bottle disappear.
Students also viewed these Sciences questions
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Consider an aqueous solution with a high concentration of micelles (Box 25-1) and relatively low concentrations of the fluorescent molecule pyrene and a quencher (cetylpyridinium chloride, designated...
-
Summarize the given text by making the notes of main points. To describe pascal's law * To state Bernoulli's principle and explain 00002.1 1INTRODUCTION Fluid is the state of a substance having the...
-
Consider the exchange rate between South Korea and Costa Rica. Typically, exchange rates vary over time, sometimes quite dramatically. The scenarios present various changes that may affect the...
-
A coal-fired boiler produces superheated steam steadily at 1 MPa, 500C from the feed water which enters the boiler at 1 MPa, 50C. For a flow rate of 10 kg/s, determine. (a) The rate of heat transfer...
-
An option's premium is 1.50 and has a delta of 40 with an underlying future at 100. If the underlying future moved from 100 to 102, what would the option's new premium be based on delta?
-
Graph the expectation function for the logistic growth model (12.34) for \(\theta_{1}=10, \theta_{2}=2\), and values of \(\theta_{3}=0.25,1,2,3\), respectively. Overlay these plots on the same set of...
-
Recall that in exercise 40 the personnel director for Electronics Associates developed the following estimated regression equation relating an employees score on a job satisfaction test to length of...
-
Research articles regarding marketing to Baby Boomers (find at least 3). (This will be your Target Market Demographics Age Range). Please be specific and find as much information as you can about...
-
Wood-Mode Company is involved in the design, manufacture, and installation of various types of wood products for large construction projects. Wood-Mode recently completed a large contract for Stadium...
-
An automotive fuel injector dispenses a fine spray of gasoline into the automobile cylinder, as shown in the bottom drawing here. When an injector gets clogged, as shown in the top drawing, the spray...
-
Proteins can be precipitated out of aqueous solution by the addition of an electrolyte; this process is called salting out the protein. (a) Do you think that all proteins would be precipitated out to...
-
What is meant by the terms (a) Capital call, (b) Deal flow, and (c) Due diligence?
-
Conduct a SWOT analysis of the University of Alabama Athletic Department with regards to building a new basketball arena on campus.
-
What characteristics of a commonweal organization would likely conflict with the culture of a typical business organization that contracts to provide a public service?
-
Greeks: IBM stock is currently priced at $50. IBM pays no dividend within the next 6 months. Consider a European call and a European put, both struck at $50, on IBM stock, both with maturity 7 = 3...
-
What are the pros and cons of allocating your budget across many smaller influencers (e.g. a portfolio of nano-/micro-influencers, think <100k costs)
-
What transformations occur in the social status of immigrant workers upon securing stable employment?
-
Yuki (age 45 at year-end) has been contributing to a traditional IRA for years (all deductible contributions) and her IRA is now worth $50,000. She is trying to decide whether she should roll over...
-
A company pledges their receivables so they may Multiple Choice Charge a factoring fee. Increase sales. Recognize a sale. Collect a pledge fee. Borrow money. Failure by a promissory notes' maker to...
-
Under special conditions, sulfur reacts with anhydrous liquid ammonia to form a binary compound of sulfur and nitrogen. The compound is found to consist of 39.6% S and 30.4% N. Measurements of its...
-
Write the electron configuration for silicon. Identify the valence electrons in this configuration and the non valence electrons. From the standpoint of chemical reactivity, what is the important...
-
A common form of elemental phosphorus is the tetrahedral P4 molecule, where all four phosphorus atoms are equivalent: At room temperature phosphorus is a solid. (a) Do you think there are any...
-
Determine the material inventory balance at the end of may? Received Issued Receiving Received Materials Report Number Received Quantity Unit Price Requisition Number Issued Quantity Issued Balance...
-
During October 2 0 2 3 , Fern Field Farms, Inc. received $ 1 0 , 0 0 0 from customers in exchange for fruit and vegetables. During the same month, the company paid $ 2 , 0 0 0 to employees, $ 5 0 0...
-
Given data below answer the question. Cash Accounts receivable $ 10,200 Cash dividends 15,200 Consulting revenue Office supplies 3,550 Rent expense $ 2,340 15,200 3,910 Office equipment 18,310 Land...

Study smarter with the SolutionInn App


