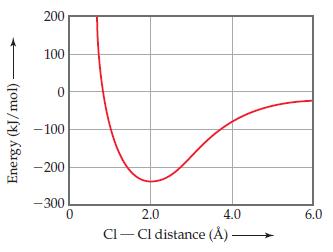
The following plot shows the potential energy of two Cl atoms as a function of the distance
Question:
The following plot shows the potential energy of two Cl atoms as a function of the distance between them.
(a) If the two atoms are very far away from each other, what is their potential energy of interaction?
(b) We know that the Cl2 molecule exists. What is the approximate bond length and bond strength for the Cl–Cl bond in Cl2 from this graph?
(c) If the Cl2 molecule is compressed under higher and higher pressure, does the Cl–Cl bond become stronger or weaker?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus
Question Posted:





