The table below shows some physical properties of halogenated liquids. Which of the following statements best explains
Question:
The table below shows some physical properties of halogenated liquids.
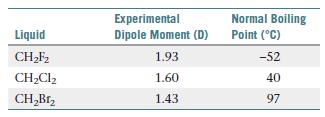
Which of the following statements best explains these data?
(a) The larger the dipole moment, the stronger the intermolecular forces, and therefore the boiling point is lowest for the molecule with the largest dipole moment.
(b) The dispersion forces increase from F to Cl to Br; since the boiling point also increases in this order, the dispersion forces must make a far greater contribution to intermolecular interactions than dipole–dipole interactions.
(c) The trend in electronegativity is F > Cl > Br; therefore, the most ionic compound (CH2F2) has the lowest boiling point, and the most covalent compound (CH2Br2) has the highest boiling point.
(d) Boiling point increases with molecular weight for these nonpolar compounds.
Step by Step Answer:

Chemistry The Central Science
ISBN: 978-0134414232
14th Edition
Authors: Theodore Brown, H. LeMay, Bruce Bursten, Catherine Murphy, Patrick Woodward, Matthew Stoltzfus





