The pH scale is used to measure the acidity or alkalinity of a solution. The scale ranges
Question:
The pH scale is used to measure the acidity or alkalinity of a solution. The scale ranges from 0 to 14. A neutral solution, such as pure water, has a pH of 7. An acid solution has a pH less than 7 and an alkaline solution has a pH greater than 7. The lower the pH below 7, the more acidic is the solution. Each whole-number decrease in pH represents a tenfold increase in acidity.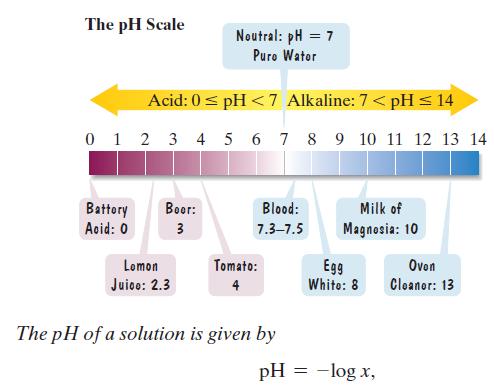 where x represents the concentration of the hydrogen ions in the solution, in moles per liter. Use the formula to solve Exercises 119–120. Express answers as powers of 10.
where x represents the concentration of the hydrogen ions in the solution, in moles per liter. Use the formula to solve Exercises 119–120. Express answers as powers of 10.
a. The figure indicates that lemon juice has a pH of 2.3. What is the hydrogen ion concentration?
b. Stomach acid has a pH that ranges from 1 to 3. What is the hydrogen ion concentration of the most acidic stomach?
c. How many times greater is the hydrogen ion concentration of the acidic stomach in part (b) than the lemon juice in part (a)?
Step by Step Answer:






