(a) Suppose there is heat transfer of 40.00 J to a system, while the system does 10.00...
Question:
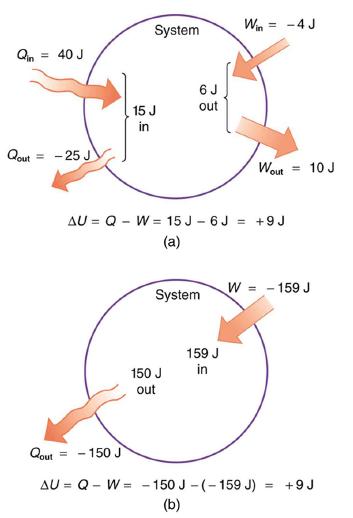
(a) Suppose there is heat transfer of 40.00 J to a system, while the system does 10.00 J of work. Later, there is heat transfer of 25.00 J out of the system while 4.00 J of work is done on the system. What is the net change in internal energy of the system?
(b) What is the change in internal energy of a system when a total of 150.00 J of heat transfer occurs out of (from) the system and 159.00 J of work is done on the system? (See Figure 15.4).
Strategy
In part (a), we must first find the net heat transfer and net work done from the given information. Then the first law of thermodynamics (ΔU = Q-W) can be used to find the change in internal energy. In part (b), the net heat transfer and work done are given, so the equation can be used directly.
Data given in Figure 15.4
Step by Step Answer:






