Calculate the activity due to 14 C in 1.00 kg of carbon found in a living organism.
Question:
Calculate the activity due to 14C in 1.00 kg of carbon found in a living organism. Express the activity in units of Bq and Ci.
Strategy
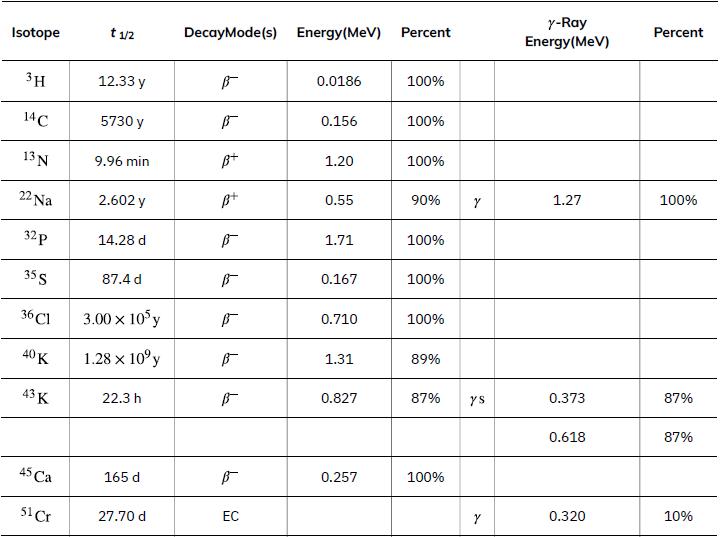
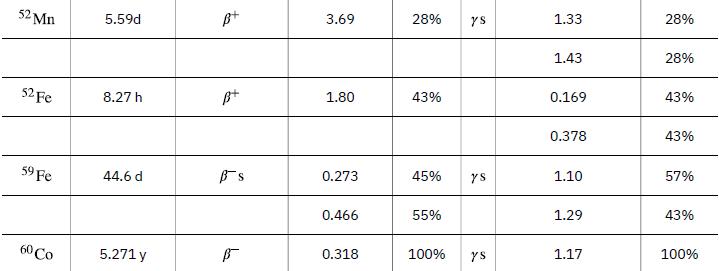
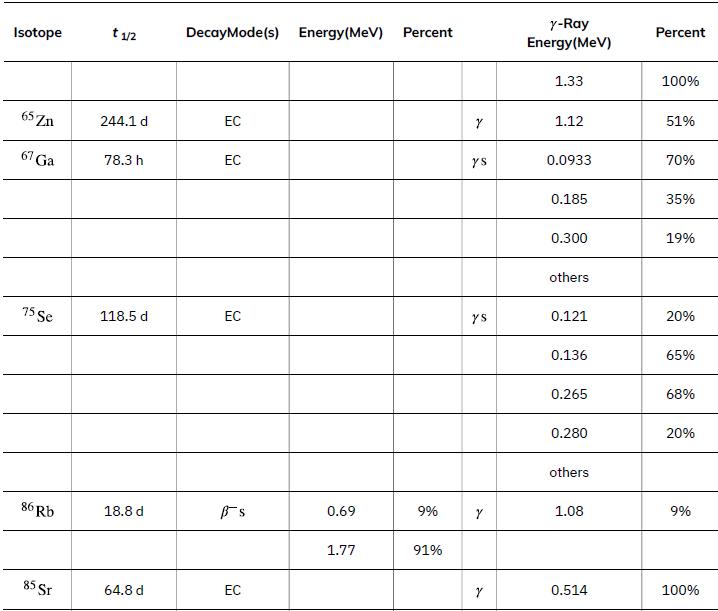
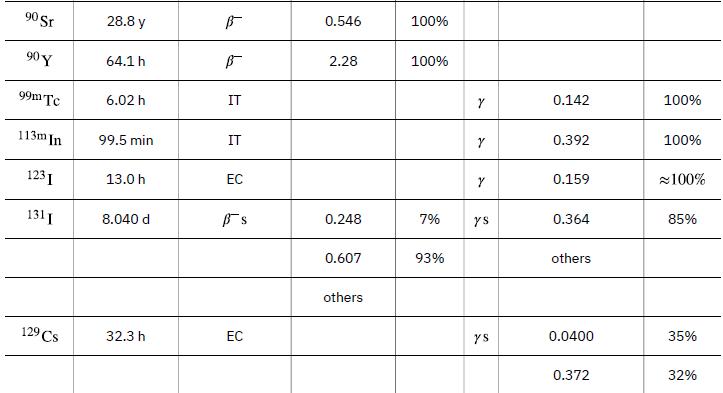
To find the activity R using the equation ![]() we must know N and t1/2. The half-life of 14C can be found in Appendix B, and was stated above as 5730 y. To find N, we first find the number of 12C nuclei in 1.00 kg of carbon using the concept of a mole. As indicated, we then multiply by 1.3x10-12 (the abundance of 14C in a carbon sample from a living organism) to get the number of 14C nuclei in a living organism.
we must know N and t1/2. The half-life of 14C can be found in Appendix B, and was stated above as 5730 y. To find N, we first find the number of 12C nuclei in 1.00 kg of carbon using the concept of a mole. As indicated, we then multiply by 1.3x10-12 (the abundance of 14C in a carbon sample from a living organism) to get the number of 14C nuclei in a living organism.
Data from Appendix B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





