Figure 30.37 shows the energy-level diagram for neon. (a) Verify that the energy of the photon emitted
Question:
Figure 30.37 shows the energy-level diagram for neon.
(a) Verify that the energy of the photon emitted when neon goes from its metastable state to the one immediately below is equal to 1.96 eV.
(b) Show that the wavelength of this radiation is 633 nm.
(c) What wavelength is emitted when the neon makes a direct transition to its ground state?
Data from Figure 30.37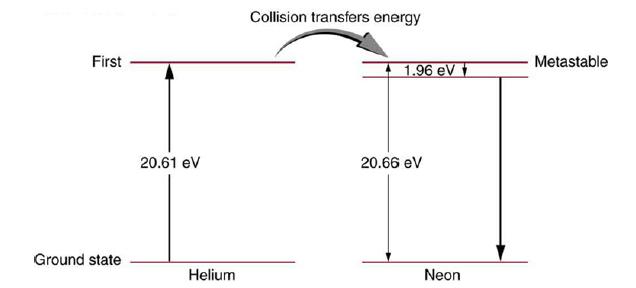
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





