Find the energy emitted in the - decay of 60 Co. Strategy and Concept As in
Question:
Find the energy emitted in the β- decay of 60Co.
Strategy and Concept
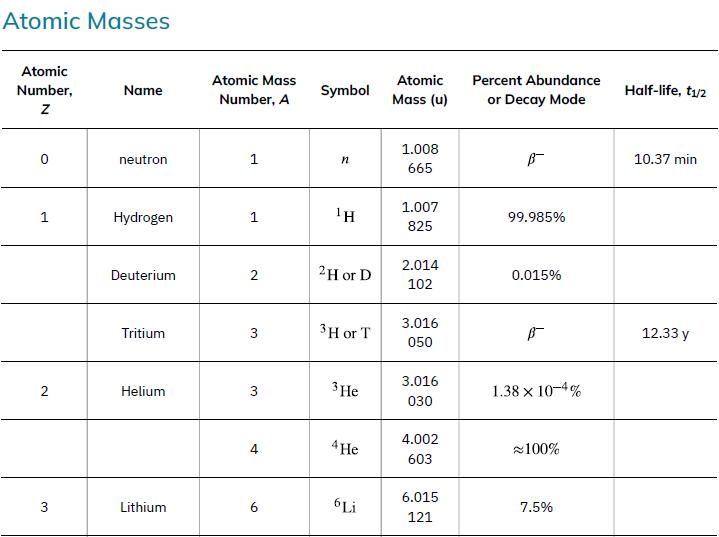
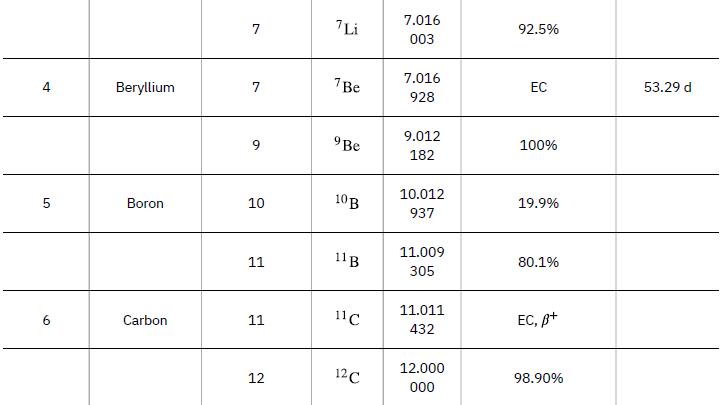
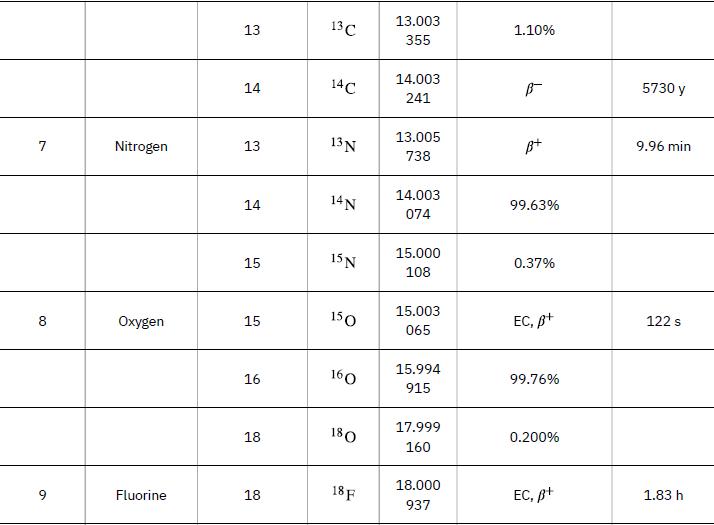
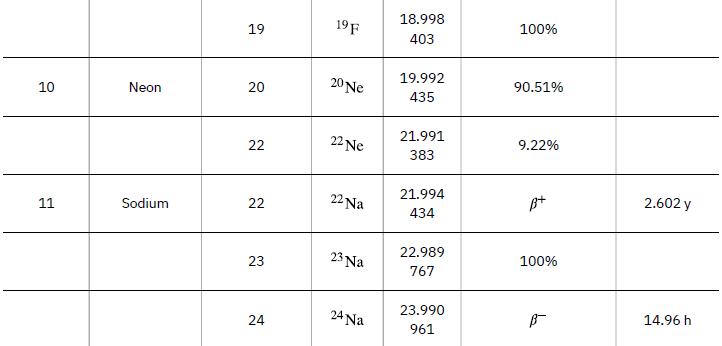
As in the preceding example, we must first find Δm, the difference in mass between the parent nucleus and the products of the decay, using masses given in Appendix A. Then the emitted energy is calculated as before, using E = (Δm)c2. The initial mass is just that of the parent nucleus, and the final mass is that of the daughter nucleus and the electron created in the decay. The neutrino is massless, or nearly so. However, since the masses given in Appendix A are for neutral atoms, the daughter nucleus has one more electron than the parent, and so the extra electron mass that corresponds to the β- is included in the atomic mass of Ni. Thus,
![]()
Data from Appendix A
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





