({ }^{235} mathrm{U}) decays to ({ }^{207} mathrm{~Pb}) via the decay series shown in Figure 30.15. The...
Question:
\({ }^{235} \mathrm{U}\) decays to \({ }^{207} \mathrm{~Pb}\) via the decay series shown in Figure 30.15. The first decay in the chain, that of \({ }^{235} \mathrm{U}\), has a half-life of \(7.0 \times 10^{8}\) years. The subsequent decays are much more rapid, so we can take this as the half-life for the complete decay of \({ }^{235} \mathrm{U}\) to \({ }^{207} \mathrm{~Pb}\). Certain minerals exclude lead but not uranium from their crystal structure, so when the minerals form they have no lead, only uranium. As time goes on, the uranium decays to lead, so measuring the ratio of lead atoms to uranium atoms allows investigators to determine the ages of the minerals. If a sample of a mineral contains 3 atoms of \({ }^{207} \mathrm{~Pb}\) for every 1 atom of \({ }^{235} \mathrm{U}\), how many years ago was it formed?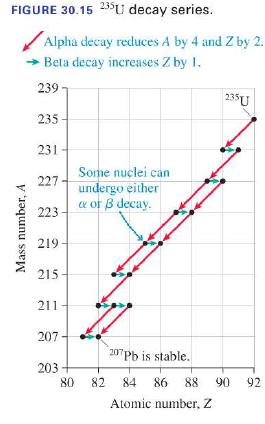
Step by Step Answer:

College Physics A Strategic Approach
ISBN: 9780321907240
3rd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field





