A mass spectrometer similar to the one in Figure P24.67 is designed to analyze biological samples. Molecules
Question:
A mass spectrometer similar to the one in Figure P24.67 is designed to analyze biological samples. Molecules in the sample are singly ionized, then they enter a \(0.80 \mathrm{~T}\) uniform magnetic field at a speed of \(2.3 \times 10^{5} \mathrm{~m} / \mathrm{s}\). If a molecule has a mass 85 times the mass of the proton, what will be the approximate distance between the points where the ion enters and exits the magnetic field?
A. \(25 \mathrm{~cm}\)
B. \(50 \mathrm{~cm}\)
C. \(75 \mathrm{~cm}\)
D. \(100 \mathrm{~cm}\)
If you have a sample of unknown composition, a first step at analysis might be a determination of the masses of the atoms and molecules in the sample. A mass spectrometer to make such an analysis can take various forms, but for many years the best technique was to determine the masses of ionized atoms and molecules in a sample by observing their circular paths in a uniform magnetic field, as illustrated in Figure P24.67. A sample to be analyzed is vaporized, then singly ionized. The ions are accelerated through an electric field, and ions of a known speed selected. These ions travel into a region of uniform magnetic field, where they follow circular paths. An exit slit allows ions that have followed a particular path to be counted by a detector, producing a record of the masses of the particles in the sample.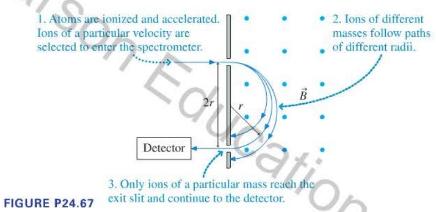
Step by Step Answer:

College Physics A Strategic Approach
ISBN: 9780321907240
3rd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field





