Molecules of carbon monoxide are permanent electric dipoles due to unequal sharing of electrons between the carbon
Question:
Molecules of carbon monoxide are permanent electric dipoles due to unequal sharing of electrons between the carbon and oxygen atoms. Figure P20.37 shows the distance and charges. Suppose a carbon monoxide molecule with a horizontal axis is in a vertical electric field of strength \(15,000 \mathrm{~N} / \mathrm{C}\).
a. What is the magnitude of the net force on the molecule?
b. What is the magnitude of the torque on the molecule?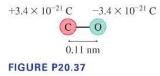
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics A Strategic Approach
ISBN: 9780321907240
3rd Edition
Authors: Randall D. Knight, Brian Jones, Stuart Field
Question Posted:





