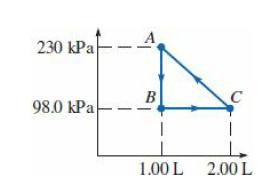
An ideal monatomic gas is taken through the cycle in the PV diagram. (a) If there are
Question:
An ideal monatomic gas is taken through the cycle in the PV diagram.
(a) If there are 0.0200 mol of this gas, what are the temperature and pressure at point C?
(b) What is the change in internal energy of the gas as it is taken from A to B?
(c) How much work is done on this gas per cycle?
(d) What is the total change in internal energy of this gas in one cycle?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

College Physics With An Integrated Approach To Forces And Kinematics
ISBN: 978-1260547719
5th Edition
Authors: Alan Giambattista
Question Posted:





