The amphoteric reaction between two water molecules is endothermic, which means the reaction requires the input of
Question:
The amphoteric reaction between two water molecules is endothermic, which means the reaction requires the input of heat energy in order to proceed: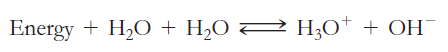 Thewarmer the water, the more heat energy is available for this reaction, and the more hydronium and hydroxide ions are formed.
Thewarmer the water, the more heat energy is available for this reaction, and the more hydronium and hydroxide ions are formed.
(a) Which has a lower pH: pure water that is hot or pure water that is cold?
(b) Is it possible for water to be neutral but to have a pH less than or greater than 7.0?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Conceptual Physical Science
ISBN: 978-0134060491
6th edition
Authors: Paul G. Hewitt, John A. Suchocki, Leslie A. Hewitt
Question Posted:





