Values of K 13.3 and K 13.5 can deviate significantly from unity; typical values for K 13.3
Question:
Values of Kϕ13.3 and Kϕ13.5 can deviate significantly from unity; typical values for Kϕ13.3 range from 0.7 to 0.9 at a pressure of 7.5 MPa and typical MSR temperatures from 200°C to 320°C, while values for Kϕ13.5 range from 0.8 to 0.95. Estimates of Kϕi may be obtained from an equation of state, but such calculations are beyond the scope of the present discussion. Furthermore, computational alternatives often require a choice between simplified and more realistic, but complex, models. An instance that illustrates this situation is estimation Kϕ13.3 of and Kϕ13.5 for use in determining reaction equilibrium constants.
a. What physical model is invoked if it is assumed that Kϕ13.3 = Kϕ13.5 = 1.0?
b. In the calculations of Problem 13.15, Kϕ13.3 and Kϕ13.5 were taken to be 1.0. Determine the significance of the ideal gas assumptions by reestimating the conversions of CO and H2 first with
Kϕ13.3 = 0.7 and Kϕ13.5 = 1.0, then with Kϕ13.3 = 1.0 and Kϕ13.5 = 0.8, and finally with Kϕ13.3 = 0.7 and Kϕ13.5 = 0.8.
c. From the preceding parts of this problem, what would be your decision (and why?) regarding inclusion of methods to estimate Kϕ13.3 and Kϕ13.5 in the process analysis?
Problem 13.15
As covered in the Process Description, there are three primary reactions that occur in the MSR. These are given by Equations 13.3, 13.4, and 13.5. However, determination of chemical equilibrium among the species H2, CO, CO2, H2O, and CH3OH involves only two of the three reactions because each reaction is a linear combination of the other two. Cherednichenko gives an approximation for the equilibrium relationships in Equation 13.3 (see Problem 13.13) and in Equation 13.5:
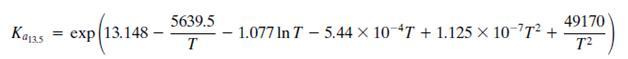
The equilibrium Ka13.5 constant is defined by the relationship
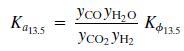
Where T is in kelvin and Kϕ13.5 accounts for nonideal behavior of the gas phase.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





