You are to simulate a single-phase reactor in which a single reaction takes place. The reaction has
Question:
You are to simulate a single-phase reactor in which a single reaction takes place. The reaction has the general form.
In this equation Ai is the ith reactant or product and vi is the stoichiometric coefficient of this species (negative for reactants and positive for products). It is also convenient to define vi for each inert species in the feed to the reactor, assigning it a value of 0. The inputs to the module are the feed stream flow rate, composition, and temperature, the fractional conversion of one of the reactants, and the product stream temperature. The module is to calculate the product stream component flow rates and the required heat transfer to the reactor.
The module equations should be written in terms of the following variables:
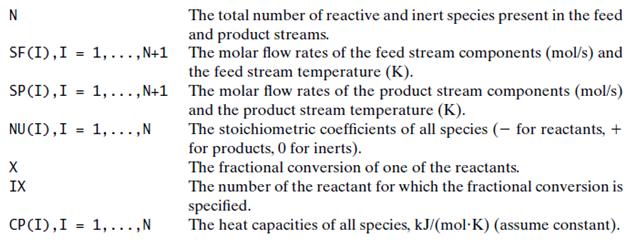

Write the equations you would use to calculate and the first components of SP from specified values of all of the other variables defined above.
Write a spreadsheet to perform the calculations of part (a) for a reactor in which propane flowing at a rate of 270 standard cubic meters per hour is burned with 20% excess air. The combined stream enters the reactor at 423 K and the stack gas leaves at 1050 K. Ninety percent of the propane fed is converted, and no CO is contained in the stack gas. Take the heat capacity of each species to be its value at 700 K as calculated from Table B.2 [so that, for example, CP(1) = 0.1431 kJ/(mol ∙ K), where (1) refers to propane]. After you have performed the calculations and recorded the output variable values, use the spreadsheet to generate a plot of Q versus stack gas temperature and briefly explain why the plot looks the way it does.
(c) Use an equation-solving program to perform the calculations outlined in part (b).
(d) Write a computer subprogram REACTS to implement the procedure of part (a). The subprogram arguments should be SF, SP, NU, N, X, IX, and Q. The arrays CP and HF should either be transmitted as additional arguments or via a COMMON or GLOBAL statement. Write and run a calling program that defines the input variables, calls the subprogram, and prints out the required out-put variables for the test case of part (b). Number the species involved in the process as follows: 1—C3H8, 2-O2, 3—N2, 4—CO2, 5—H2O. For example, NU(1) = -1, NU(2) = -5, SF(1) = 3.348, SF(3) = 75.54, SF(6) = 423, and SP(6) = 1050. (Verify these values as part of your problem solution.)
Table B.2
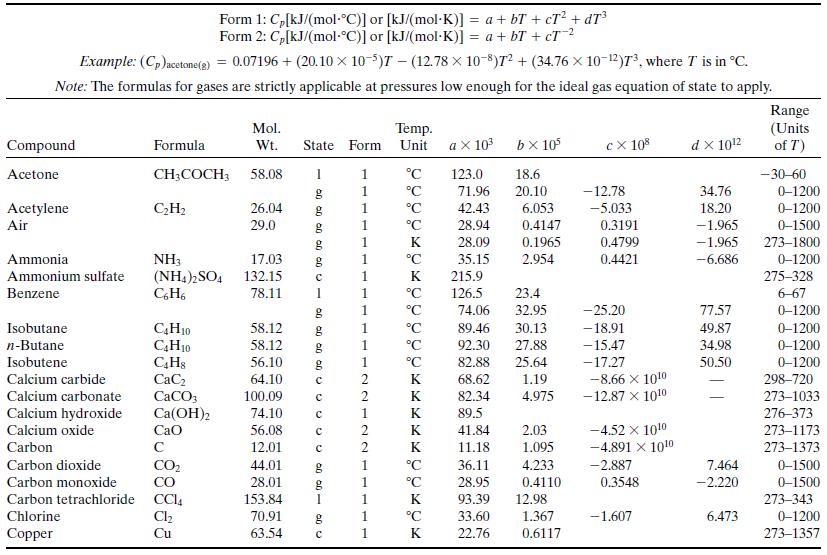
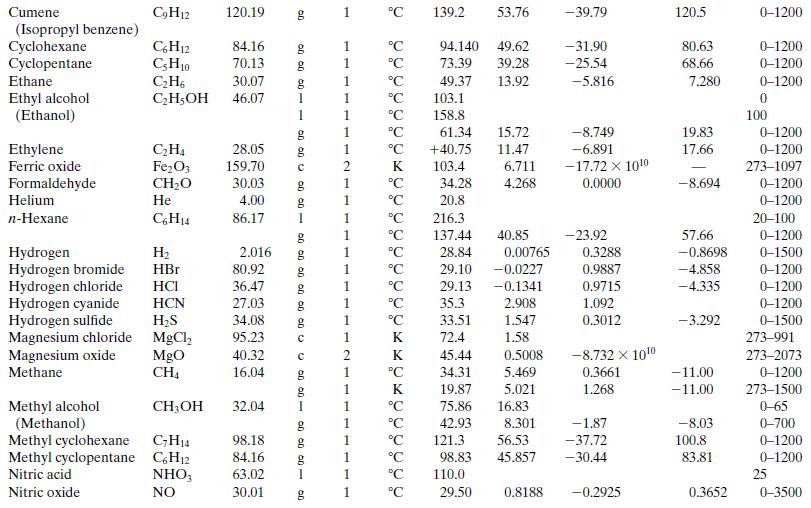
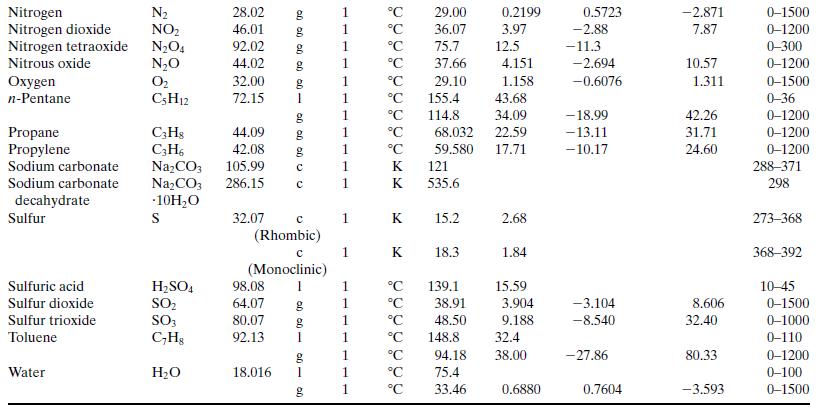
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau




