Water molecules are said to reorganize into a cagelike structure around hydrophobic compounds (e.g., see Figure 119).
Question:
Water molecules are said “to reorganize into a cagelike structure” around hydrophobic compounds (e.g., see Figure 11–9). This seems paradoxical because water molecules do not interact with the hydrophobic compound. So how could they “know” about its presence and change their behavior to interact differently with one another? Discuss this argument and, in doing so, develop a clear concept of what is meant by a cagelike” structure. How does it compare to ice? Why would this cagelike structure be energetically unfavorable?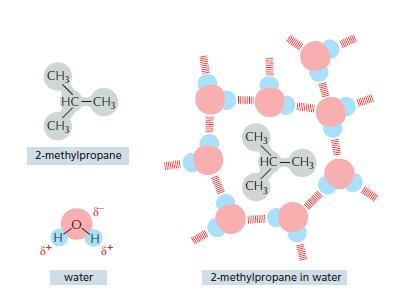
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Essential Cell Biology
ISBN: 9780393680362
5th Edition
Authors: Bruce Alberts, Karen Hopkin, Alexander Johnson, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Question Posted:





