Show that it follows directly from the equation of state, P = RT/, that the internal energy
Question:
Show that it follows directly from the equation of state, P = RT/α, that the internal energy of an ideal gas is a function of temperature only.
Solution: from (??) and p = RT/α, we have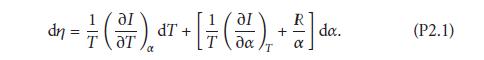
But, mathematically,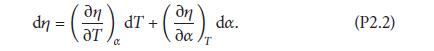
Equating the coefficients of dT and dα in these two expressions gives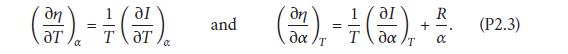
Noting that ∂2η/(∂α∂T) = ∂2η/(∂T∂α) we obtain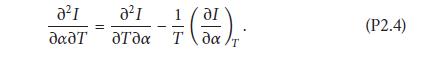
Thus, (∂T/∂α)T = 0. Because, in general, the internal energy may be considered either a function of temperature and density or temperature and pressure, this proves that for an ideal gas the internal energy is a function only of temperature.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Essentials Of Atmospheric And Oceanic Dynamics
ISBN: 9781107692794
1st Edition
Authors: Geoffrey K. Vallis
Question Posted:





