A horizontal chemical vapor deposition (CVD) reactor similar to the configuration shown in Example 3, Figure 28.6
Question:
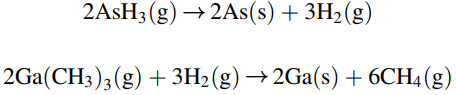
If the reactant gas is considerably diluted in H2 gas, then the mass transfer of each species in the H2 carrier gas can be treated separately. These surface reactions are considered to be very rapid, and so the mass transfer of the gaseous reactants to the surface of the wafer limits the rate of GaAs thin film formation. In the present process, a 15 cm × 15 cm square silicon wafer is positioned at the leading edge of the susceptor plate. The process temperature is 800K, and the total system pressure 101.3 kPa (1.0 atm). The feed gas delivered to the reactor results in a bulk linear velocity of 100 cm/s. The composition of arsine and trimethylgallium in the feed gas are both 0.10 mole%, which is very dilute. You may assume that the amount of arsine and trimethylgallium delivered with the feed gas is much higher than the amount of arsine and trimethylgallium consumed by the reactions, so that the concentration of these reactants in the bulk gas phase is essentially constant down the length of the reactor. You may also assume that the surface-reaction rates are instantaneous relative to the rates of mass transfer, so that the gas- phase concentrations of both arsine vapor and trimethylgallium vapor at the surface of the wafer are essentially zero. The binary gas phase diffusion coefficient of trimethylgallium in H2 is 1.55 cm2/s at 800 K and 1.0 atm.
a. What are the average mass-transfer rates for arsine and trimethylgallium over the whole wafer?
b. Based on the ratio of the arsine and trimethylgallium mass transfer rates, what is the composition of the GaAs compos ite thin film €“ e.g., the molar composition of gallium (Ga) and arsenic (As) in the solid? How could the feed-gas composition be adjusted so that the molar ratio of Ga to As within the solid thin film is 1:1?
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





