Question:
Carbon dioxide (CO
2) from waste sources is a sustain able feed stock for chemicals production if new technologies can be developed to carry out the reduction of CO
2, the most oxidized form of carbon, to a more reactive molecule. Recently, solar-energy driven reactor concepts have emerged that harness the sun€™s energy to sustain ably drive the thermo-catalytic reduction of CO
2to reactive CO over a Ceria catalyst at high temperatures. A highly simplified version of this concept is provided in the figure in the next column, which is operated at 800
°C and 1.0 atm. The diameter of the cylindrical reactor is 10.0 cm, the thickness of the porous catalyst lining the base of the reactor is 1.0 cm, and the gas space above the porous catalyst layer can be considered well mixed. Pure CO
2is fed into the reactor and diffuses into the porous catalyst layer, which drives the reaction CO
2(g) †’ CO(g) + 1/2O
2(g), which is approximated as first order with rate constant of k = 6.0 s
-1at 800
°C. The effective diffusion coefficient of CO
2in the gas mixture within the porous catalyst is 0.40 cm
2/s at 1.0 atm and 800
°C.
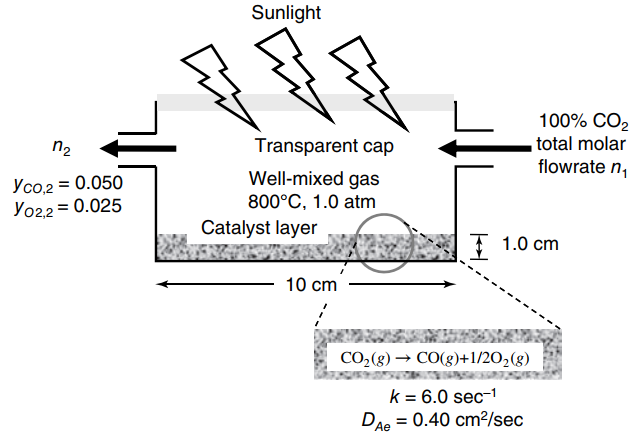
a. It is desired to achieve 5.0 mole% CO in the exiting outlet gas. What is the molar flow rate exiting the reactor (n
2), in units of gmole/min? What is the inlet molar flow rate of CO
2?
b. What is the mole fraction of CO
2on the back side of the catalyst layer?
Transcribed Image Text:
Sunlight 100% CO2 total molar flowrate n, Transparent cap n2 Well-mixed gas 800°C, 1.0 atm Yco,2 = 0.050 Yo2,2 = 0.025 Catalyst layer I 1.0 cm 10 cm CO,(g) → CO(g)+1/2O2(g) k = 6.0 sec-1 DAe = 0.40 cm²/sec








