Exit gas from an amination reactor contains 10 mole% ammonia (NH 3 ) vapor in a nitrogen
Question:
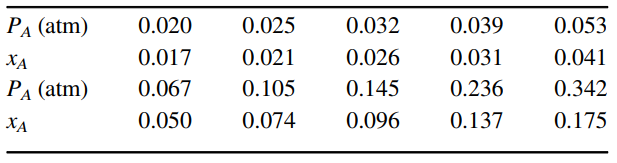
Exit gas from an amination reactor contains 10 mole% ammonia (NH3) vapor in a nitrogen (N2) carrier gas. This gas mixture is fed into the bottom of a packed tower at a molar flow rate of 2.0 kgmole/s. The NH3will be absorbed into water at neutral pH within a packed tower operating in counter current flow. The gas exiting the top of the tower contains 2.0 mole% NH3. Water containing 1.0 mole% of residual dissolved ammonia enters the top of the tower at a molar flow rate of 3.0 kgmole/s. The tower is packed with 1.0-inch ceramic Intalox saddles, and operates at 20?C and 2.5 atm total system pressure. At these conditions, the mass density of the feed gas is 2.8 kg/m3, the density of the liquid is 1000 kg/m3, and the viscosity of the liquid is 1.0 cP (0.001 kg/m ? s). Equilibrium distribution data for NH3in water at 30?C is provided in the following table.a. Specify the molar flow rate and mole fraction composition of all terminal streams for the process. Will the tower operate as intended? Investigate this issue by plotting the operating line relative to the equilibrium line in mole fraction and mole ratio coordinates.
b. What is the minimum tower diameter at the ??flooding?? condition, and the tower diameter at 50% of the flooding condition?c. What are the overall mass-transfer driving forces (yA - y*A) at the top and bottom of the tower, and the log-mean driving force?Equilibrium distribution data for NH3-water at 30?C (A = NH3):
The word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Step by Step Answer:

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster





