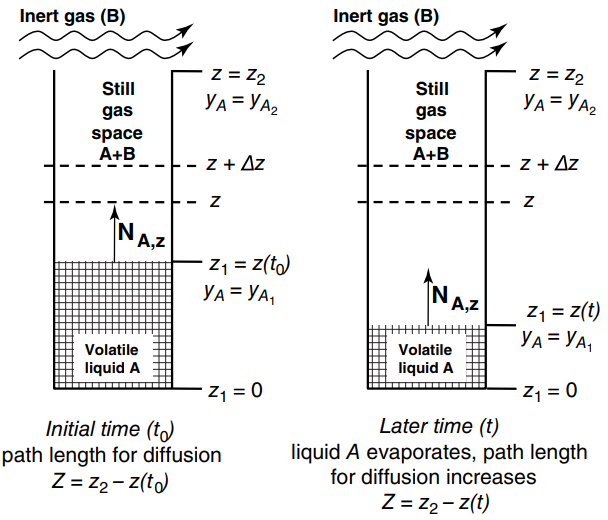
The Arnold Diffusion Cell shown in Figure 26.5 is a simple device used to measure gas-phase diffusion
Question:
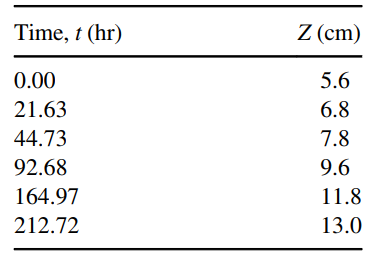
a. Manipulate the data shown in the table above so that it can be plotted as a straight line. Statistically estimate the slope of this line by least-squares linear regression, and then use the slope to estimate the diffusion coefficient of acetone in air.
b. Compare the result in part (a) to an estimate of diffusion coefficient of acetone in air by a suitable correlation given in Chapter 24.
Potentially useful data: The molecular weight of acetone (MA) is 58 g/gmole; the density of liquid acetone (ÏA,liq) is 0.79 g/cm3; the vapor pressure of acetone (PA*) at 20.9°C is 193 mm Hg.
Figure 26.5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals Of Momentum Heat And Mass Transfer
ISBN: 9781118947463
6th Edition
Authors: James Welty, Gregory L. Rorrer, David G. Foster
Question Posted:





